Elucidating the mechanisms of nickel compound uptake: a review of particulate and nano-nickel endocytosis and toxicity - PubMed (original) (raw)
Review
Elucidating the mechanisms of nickel compound uptake: a review of particulate and nano-nickel endocytosis and toxicity
Alexandra Muñoz et al. Toxicol Appl Pharmacol. 2012.
Abstract
Nickel (Ni) is a worldwide pollutant and contaminant that humans are exposed to through various avenues resulting in multiple toxic responses - most alarming is its clear carcinogenic nature. A variety of particulate Ni compounds persist in the environment and can be distinguished by characteristics such as solubility, structure, and surface charge. These characteristics influence cellular uptake and toxicity. Some particulate forms of Ni are carcinogenic and are directly and rapidly endocytized by cells. A series of studies conducted in the 1980s observed this process, and we have reanalyzed the results of these studies to help elucidate the molecular mechanism of particulate Ni uptake. Originally the process of uptake observed was described as phagocytosis, however in the context of recent research we hypothesize that the process is macropinocytosis and/or clathrin mediated endocytosis. Primary considerations in determining the route of uptake here include calcium dependence, particle size, and inhibition through temperature and pharmacological approaches. Particle characteristics that influenced uptake include size, charge, surface characteristics, and structure. This discussion is relevant in the context of nanoparticle studies and the emerging interest in nano-nickel (nano-Ni), where toxicity assessments require a clear understanding of the parameters of particulate uptake and where establishment of such parameters is often obscured through inconsistencies across experimental systems. In this regard, this review aims to carefully document one system (particulate nickel compound uptake) and characterize its properties.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1
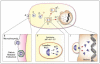
Types of Endocytosis In general endocytic mechanisms can be roughly divided into two categories depending on whether the uptake substrate consists mainly of fluids and solutes (pinocytosis) or whether it is composed of large particles (phagocytosis). Within pinocytosis there is a further division depending on whether the mechanism is dynamin dependent (clathrin and caveolar) or dynamin independent (non clathrin/noncaveolar, lipid raft-mediated, macropinocytosis). Figure adapted from Mercer and Helenius, 2009. Reprinted with permission of Nature Cell Biology
Figure 2
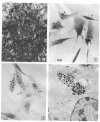
Cells Endocytizing Particles Figure 2-1 demonstrates the Ni3S2 induced morphological transformation in SHE cells. Cells exhibit disordered growth and criss-crossing. These disordered cells grow in soft agar and form tumors in athymic nude mice. Figure 2-2 is a light microscope photograph of SHE cells with arrows indicating the location of endocytized Ni3S2 particles which appear as black dots. Figure 2-3 is a light microscope photograph of CHO cells that have endocytized Ni3S2 particles with arrows indicating the location of the particles which appear as black dots are visibly encapsulated in a vesicle. Figure 2-4 is an electron micrograph of a CHO cell with endocytized Ni3S2 particles contained in a vesicle. Images from Costa and Mollenhauer 1980b. Reprinted with the permission of Cancer Research.
Figure 3
Image of Cells Endocytizing Particles Endocytosis of crystalline Ni3S2 particles. Images were recorded on videotape at 18/1 time lapse and photographs of the film were taken with Polaroid film. The total time lapse from images 3A-3H is 12 minutes. Ni3S2 particles appear white under phase contrast in these images. In 3a (0min) two particles are bound to the CHO cell surface. In panels A (0 min), E (6 min), and H (12 min) the particle indicated by the white long arrow is endocytized over the time course while the particle indicated by the white short arrow remains affixed to the cell surface. Images from Evans et al. 1982. Reprinted with permission of Cancer Research.
Figure 4
Proposed Model of Particulate Nickel Uptake and Intracellular Distribution The nickel particle, crystalline Ni3S2 affixes at the cell surface (A). We hypothesize that the particle enters via macropinocytosis and/or clathrin mediated endocytosis - where the different forms of uptake may be related to the size of the particle (B). In macropinocytosis the membrane exhibits ruffling prior to uptake, a feature that was frequently observed during Ni3S2 endocytosis. In clathrin-mediated endocytosis the membrane undergoes a morphological change via invagination and forms a membrane pit. A number of proteins are involved in CME, pictured here are the clathrin proteins that stabilize the pit curvature and the dynamin that aggregates at the neck prior to scission. The endocytized particle moves via saltatory motion towards the nucleus inside some form of vesicle (the specific form will vary in accordance with the form of endocytosis - macropinosome or clathrin coated vesicle) (C). Lysosomes then interact with the vesicle in a process of lysosomal attack (D). These interactions often lead to lysosomal fusion (E). Once fused, the pH of the vesicle may be altered through proton pumps leading to acidification of the vesicle and the dissolution of the crystalline Ni3S2 particle. This process produces high concentrations of Ni2+ (F). In some cases the Ni2+ may exit the vesicle into the cytoplasm where they can interact with biomolecules (G); while in other cases the vesicles will continue to travel towards the nucleus where they will aggregate at the nuclear membrane (H). Those aggregated at the membrane will promote the transfer of Ni2+ ions into the nucleus (I). The presence of Ni2+ can lead to multiple effects including DNA condensation and the modification of epigenetic marks including increased DNA methylation and loss of histone acetylation in H2A, H2B, H3, and H4 as well as an increase in H3K9 dimethylation, H3K4 trimethylation and ubiquitylation of H2A and H2B.
Similar articles
- Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles.
Pietruska JR, Liu X, Smith A, McNeil K, Weston P, Zhitkovich A, Hurt R, Kane AB. Pietruska JR, et al. Toxicol Sci. 2011 Nov;124(1):138-48. doi: 10.1093/toxsci/kfr206. Epub 2011 Aug 9. Toxicol Sci. 2011. PMID: 21828359 Free PMC article. - Comparative pulmonary toxicity of inhaled nickel nanoparticles; role of deposited dose and solubility.
Kang GS, Gillespie PA, Gunnison A, Rengifo H, Koberstein J, Chen LC. Kang GS, et al. Inhal Toxicol. 2011 Feb;23(2):95-103. doi: 10.3109/08958378.2010.543440. Epub 2011 Jan 24. Inhal Toxicol. 2011. PMID: 21261442 - Toxicity of Nanoparticulate Nickel to Aquatic Organisms: Review and Recommendations for Improvement of Toxicity Tests.
Meyer JS, Lyons-Darden T, Garman ER, Middleton ET, Schlekat CE. Meyer JS, et al. Environ Toxicol Chem. 2020 Oct;39(10):1861-1883. doi: 10.1002/etc.4812. Epub 2020 Aug 25. Environ Toxicol Chem. 2020. PMID: 32619073 Free PMC article. Review. - Recent progress in studies of metallic nickel and nickel-based nanoparticles' genotoxicity and carcinogenicity.
Magaye R, Zhao J. Magaye R, et al. Environ Toxicol Pharmacol. 2012 Nov;34(3):644-50. doi: 10.1016/j.etap.2012.08.012. Epub 2012 Sep 6. Environ Toxicol Pharmacol. 2012. PMID: 23000472 Review.
Cited by
- Nickel-Refining Fumes Induced DNA Damage and Apoptosis of NIH/3T3 Cells via Oxidative Stress.
Wang Y, Wang SY, Jia L, Zhang L, Ba JC, Han D, Yu CP, Wu YH. Wang Y, et al. Int J Environ Res Public Health. 2016 Jun 23;13(7):629. doi: 10.3390/ijerph13070629. Int J Environ Res Public Health. 2016. PMID: 27347984 Free PMC article. - Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers.
Torres-Vanegas JD, Cruz JC, Reyes LH. Torres-Vanegas JD, et al. Pharmaceutics. 2021 Mar 22;13(3):428. doi: 10.3390/pharmaceutics13030428. Pharmaceutics. 2021. PMID: 33809969 Free PMC article. Review. - Biological and environmental interactions of emerging two-dimensional nanomaterials.
Wang Z, Zhu W, Qiu Y, Yi X, von dem Bussche A, Kane A, Gao H, Koski K, Hurt R. Wang Z, et al. Chem Soc Rev. 2016 Mar 21;45(6):1750-80. doi: 10.1039/c5cs00914f. Chem Soc Rev. 2016. PMID: 26923057 Free PMC article. Review. - Epigenetic influence of environmentally neurotoxic metals.
Ijomone OM, Ijomone OK, Iroegbu JD, Ifenatuoha CW, Olung NF, Aschner M. Ijomone OM, et al. Neurotoxicology. 2020 Dec;81:51-65. doi: 10.1016/j.neuro.2020.08.005. Epub 2020 Sep 1. Neurotoxicology. 2020. PMID: 32882300 Free PMC article. Review. - A comprehensive review on the sources, essentiality and toxicological profile of nickel.
Begum W, Rai S, Banerjee S, Bhattacharjee S, Mondal MH, Bhattarai A, Saha B. Begum W, et al. RSC Adv. 2022 Mar 23;12(15):9139-9153. doi: 10.1039/d2ra00378c. eCollection 2022 Mar 21. RSC Adv. 2022. PMID: 35424851 Free PMC article. Review.
References
- Abbracchio MP, Evans RM, Heck JD, Cantoni O, Costa M. The regulation of ionic nickel uptake and cyto-toxicity by specific amino-acids and serum components. Biological Trace Element Research. 1982;4:289–301. - PubMed
- Abbracchio MP, Heck JD, Caprioli RM, Costa M. Differences in surface-properties of amorphous and crystalline metal sulfides may explain their toxicological potency. Chemosphere. 1981;10:897–908.
- Anke M, Angelow L, Glei M, Muller M, Illing H. The biological importance of nickel in the food-chain. Fresenius J. Anal. Chem. 1995;352:92–96.
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES014454/ES/NIEHS NIH HHS/United States
- T32 ES007324/ES/NIEHS NIH HHS/United States
- R01 ES005512/ES/NIEHS NIH HHS/United States
- P30 ES000260/ES/NIEHS NIH HHS/United States
- 5T32 ES007324-12/ES/NIEHS NIH HHS/United States
- ES010344/ES/NIEHS NIH HHS/United States
- ES014454/ES/NIEHS NIH HHS/United States
- P42 ES010344/ES/NIEHS NIH HHS/United States
- ES005512/ES/NIEHS NIH HHS/United States
- ES000260/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources