Lack of kinase regulation of canonical transient receptor potential 3 (TRPC3) channel-dependent currents in cerebellar Purkinje cells - PubMed (original) (raw)
Lack of kinase regulation of canonical transient receptor potential 3 (TRPC3) channel-dependent currents in cerebellar Purkinje cells
Charmaine Nelson et al. J Biol Chem. 2012.
Abstract
Canonical transient receptor potential (TRPC) channels are widely expressed in the brain and play several roles in development and normal neuronal function. In the cerebellum, Purkinje cell TRPC3 channels underlie the slow excitatory postsynaptic potential observed after parallel fiber stimulation. In these cells TRPC3 channel opening requires stimulation of metabotropic glutamate receptor 1, activation of which can also lead to the induction of long term depression (LTD), which underlies cerebellar motor learning. LTD induction requires protein kinase C (PKC) and protein kinase G (PKG) activation, and although PKC phosphorylation targets are well established, virtually nothing is known about PKG targets in LTD. Because TRPC3 channels are inhibited after phosphorylation by PKC and PKG in expression systems, we examined whether native TRPC3 channels in Purkinje cells are a target for PKG or PKC, thereby contributing to cerebellar LTD. We find that in Purkinje cells, activation of TRPC3-dependent currents is not inhibited by conventional PKC or PKG to any significant extent and that inhibition of these kinases does not significantly impact on TRPC3-mediated currents either. Based on these and previous findings, we propose that TRPC3-dependent currents may differ significantly in their regulation from those overexpressed in expression systems.
Figures
FIGURE 1.
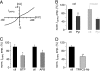
The mGluR1-mediated slow EPSC in juvenile rat cerebellar slices is TRPC3-dependent. A, shown is the current-voltage relationship of the DHPG-induced inward current (_I_DHPG) (20 μ
m
DHPG in P15 rat). B, DHPG-mediated inward current (_I_DHPG) (50 μ
m
DHPG) was significantly blocked in the presence of 10 μ
m
Pyr3 (Pyr) both in juvenile rat (P12–14; left pair of black bars; n = 5 for control (ctl) and 6 for Pyr3) and in juvenile mice (P12–14; r_ight pair of gray bars_; n = 3 for both control and Pyr3). _I_DHPG amplitudes in the presence of Pyr3 were normalized (norm.) to average control amplitude in absence of Pyr3. There was no significant difference in the extent of Pyr3-mediated block between rat and mouse (p = 0.3484; unpaired Student's t test). Preincubation in Pyr3 was 40 min. C, DHPG-mediated inward current (_I_DHPG) (50 μ
m
DHPG) was significantly blocked by 15 μ
m
BTP2 (left two columns; n = 4 for control (ctl) and 6 for BTP2 (BTP)) and by 10 μ
m
2-APB (APB; right two columns; n = 3 for each control and test; p = 0.0481, unpaired Student's t test). D, DHPG-mediated inward current (_I_DHPG) (50 μ
m
DHPG) was significantly blocked by intracellular TRPC3-antibody (TRPC3-Ab); n = 5 for control (ctl) and 4 antibody experiments. _I_DHPG amplitudes in the presence of TRPC3 antibody were normalized (norm.) to average control amplitude in the absence of the antibody. Intracellular solution was supplemented with 4 μg/ml TRPC3 antibody. Reduction in current amplitude is significant (p = 0.0013; unpaired Student's t test).
FIGURE 2.
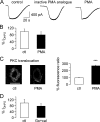
Lack of inhibition on mGluR1-mediated TRPC3 currents in rat Purkinje cells after activation of protein kinase C. A, raw traces show TRPC3 current in response to application of 50 μ
m
DHPG under control conditions (left graph) after preincubation in an inactive PMA-analog (1 μ
m
; preincubation time 13 min; middle graph) and after preincubation in the PKC activator PMA (1 μ
m
; preincubation time 14 min; right graph). B, aggregate data compare the size of the DHPG-mediated TRPC3 current (_I_DHPG) under control (ctl) conditions (including inactive PMA analog; n = 16; white column) and after exposure to PMA (n = 15; black column). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.3622, unpaired Student's t test). Average preincubation time in 1 μ
m
PMA was 15.3 ± 1.3 min (n = 15 cells) and in the inactive PMA analog, 14.1 ± 1.2 min (n = 7 cells). C, right, representative images show distribution of PKC under control conditions and after incubation with PMA (5–10 min) in 2 Purkinje cells; scale bar = 50 μm and applies to both images; left, shown is a significant increase in membrane fluorescence ratio in Purkinje cells exposed to PMA compared with control Purkinje cells, demonstrating movement and, hence, activation of PKC upon exposure to PMA (n = 9 for PMA exposed cells and n = 6 for control cells; p < 0.0001, unpaired Student's t test). D, aggregate data compare the size of the DHPG-mediated TRPC3 current under control conditions and after preincubation with 0.5 μ
m
calphostin (cal) and 1 μ
m
Go6976 (Go), or just 1 μ
m
Go6976 for 45.2 ± 2.9 min (n = 8 for control and 9 for PKC inhibitor-exposed cells; p = 0.4626, unpaired Student's t test). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
FIGURE 3.
Inhibition of TRPC3 currents by protein kinase C in expression system. A, representative fluorescence Ca2+ imaging traces recorded from HEK298 cells stably transfected with human TRPC3 show increases in intracellular Ca2+ concentration after application of 50 μ
m
OAG under control conditions (ctl, left graph), after preincubation in 1 μ
m
PMA (7 min), and after exposure of cells to PMA (n = 6 min) that had also been exposed to the PKC inhibitor Go6983 (Go) for 70 min; increases in intracellular Ca2+ concentration are monitored as changes in fluorescence ratio (Δ_F_) as described under “Experimental Procedures.” Dotted lines show fluorescence signals in cells that were considered to be non-responders. B, aggregate data show that there is a clear decrease in OAG-responsive (resp.) cells after incubation of TRPC3-transfected cells with 1 μ
m
PMA (5–10 min; black bars indicate PMA-incubated cells, and white bars indicated control cells); this decrease can be reversed by preincubating cells with 1 μ
m
Go6983 (65–75 min in Go6983; 5–10 min in PMA; gray bar). Numbers of OAG-responsive cells (cells that responded to OAG application with increase in intracellular Ca2+ concentration) are: for control conditions, 13/17 (76.4%); for PMA, 13/33 (39.4%); for PMA+Go6983, 17/24 (70.8%). C, aggregate data show that PMA treatment of TRPC3-expressing cells did not significantly interfere with timing of peak Ca2+ signal (measured as time lapsed between application of OAG and peak fluorescence signal; left three columns of the bar chart; p = 0.2621, ANOVA test). Peak Ca2+ signal (measured as peak fluorescence signal compared with base line; right three columns of the bar chart) was significantly decreased after preincubation with PMA alone (p = 0.0034; ANOVA test). White columns, control (ctl) conditions (n = 13); black columns, after exposure to PMA only (n = 13); gray columns, exposure to PMA after pretreatment with Go6983 (n = 17). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs. D, aggregate data from four control (ctl) and 5 test (PMA) HEK293 cells stably transfected with human TRPC show that TRPC3 whole cell current (WCC) responses to ATP application (100 μ
m
) are significantly inhibited after preincubation in PMA (10–15 min); p = 0.0007, unpaired Student's t test. Two cells did not respond to ATP with an inward current after PMA preincubation.
FIGURE 4.
PMA and Go6983 are active in the juvenile rat slice preparation at the concentrations used in previous slice experiments. A, representative raw data show that application of 1 μ
m
PMA to rat cerebella slices leads to an increase in mIPSC amplitude (top left, control mIPSC frequency; top right, mIPSC frequency after exposure to PMA for 11 min; data were obtained from one cell). This increase in mIPSC frequency can be inhibited by pretreatment of slices with 1 μ
m
Go6983 (Go) (bottom left) mIPSC frequency after exposure of slices to 1 μ
m
Go6983 for 40 min; bottom right, mIPSC frequency after additional exposure to 1 μ
m
PMA for 11 min; data were obtained from one cell). Top and bottom traces were recorded from two different Purkinje cells. Experiments were carried out in the absence of bicuculline. B, aggregate data show that under control (ctl) conditions application of 1 μ
m
PMA leads to a significant increase in mIPSC frequency (n = 4; p = 0.016, paired Student's t test), whereas application of 1 μ
m
PMA after preincubation to 1 μ
m
Go6983 (Go) prevents an increase in mIPSC frequency (p = 0.868 for mIPSC frequency in Go6983 compared with mIPSC frequency in Go693 and PMA, paired Student's t test).
FIGURE 5.
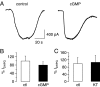
Lack of inhibition on mGluR1-mediated TRPC3 currents in rat Purkinje cells after activation of protein kinase G. A, raw traces show TRPC3 current in response to application of 50 μ
m
DHPG under control conditions (left graph) or after preincubation in 1 m
m
8-bromo-cGMP (cGMP; 43 min; right graph). B, aggregate data compare the size of DHPG-mediated TRPC3 current (_I_DHPG) under control conditions (ctl; white column; n = 6) and after preincubation with 1 m
m
8-bromo-cGMP (cGMP; preincubation for 41.4 ± 3.8 min; n = 5). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.5073, unpaired Student's t test). C, aggregate data compare the size of DHPG-mediated TRPC3 current (_I_DHPG) under control conditions (white column; n = 6) and after preincubation in the protein kinase G inhibitor KT5823 (KT, 1 μ
m
, preincubation for 62 ± 5.8 min; n = 4). Current amplitude was normalized to average control current amplitude. There was no significant difference between current amplitudes under control and test conditions (p = 0.8950, unpaired Student's t test). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
FIGURE 6.
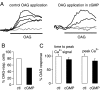
Inhibition of TRPC3 currents by protein kinase G in expression system. A, representative fluorescence Ca2+ imaging traces show increases in intracellular Ca2+ concentration in HEK298 cells stably transfected with human TRPC3 after application of 50 μ
m
OAG under control conditions (left graph) and after preincubation in 1 m
m
8-bromo-cGMP (65 min); increases in intracellular Ca2+ concentration are monitored as changes in fluorescence ratio (ΔF), as described under “Experimental Procedures.” Dotted lines show fluorescence signals in cells that were considered to be non-responders. B, aggregate data show that there is a clear decrease in OAG-responsive cells after incubation of TRPC3-transfected cells with 1 m
m
8-bromo-cGMP (60–75 min). C, aggregate data show that 8-bromo-cGMP treatment of TRPC3-expressing cells does not significantly interfere with timing of peak Ca2+ signal (measured as time lapsed between application of OAG and peak fluorescence signal; left part of the bar chart; p = 0.8694) or peak Ca2+ signal (measured as peak fluorescence signal compared with base line; right part of the bar chart; p = 0.2838, unpaired Student's t test). White columns, control (ctl; n = 38) conditions; black columns, after exposure to 8-bromo-cGMP (n = 31). All drugs under investigation were present during the OAG applications to avoid wash-out of the drugs.
Similar articles
- TRPC3 channel underlies cerebellar long-term depression.
Kim SJ. Kim SJ. Cerebellum. 2013 Jun;12(3):334-7. doi: 10.1007/s12311-013-0455-1. Cerebellum. 2013. PMID: 23408143 - Neuronal Nitric Oxide Synthase Regulates Cerebellar Parallel Fiber Slow EPSC in Purkinje Neurons by Modulating STIM1-Gated TRPC3-Containing Channels.
Gui L, Tellios V, Xiang YY, Feng Q, Inoue W, Lu WY. Gui L, et al. Cerebellum. 2024 Oct;23(5):1867-1881. doi: 10.1007/s12311-024-01683-0. Epub 2024 Mar 12. Cerebellum. 2024. PMID: 38472628 - TRPC, cGMP-dependent protein kinases and cytosolic Ca2+.
Yao X. Yao X. Handb Exp Pharmacol. 2007;(179):527-40. doi: 10.1007/978-3-540-34891-7_31. Handb Exp Pharmacol. 2007. PMID: 17217077 Review. - [Mechanisms of modification of excitatory and inhibitory inputs in various neurons of olivary-cerebellar network].
Sil'kis IG. Sil'kis IG. Zh Vyssh Nerv Deiat Im I P Pavlova. 2000 May-Jun;50(3):372-87. Zh Vyssh Nerv Deiat Im I P Pavlova. 2000. PMID: 10923375 Review. Russian.
Cited by
- TRPC3-dependent synaptic transmission in central mammalian neurons.
Hartmann J, Konnerth A. Hartmann J, et al. J Mol Med (Berl). 2015 Sep;93(9):983-9. doi: 10.1007/s00109-015-1298-7. Epub 2015 Jun 5. J Mol Med (Berl). 2015. PMID: 26041382 Review. - Cerebellar modules operate at different frequencies.
Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Zhou H, et al. Elife. 2014 May 7;3:e02536. doi: 10.7554/eLife.02536. Elife. 2014. PMID: 24843004 Free PMC article. - Alternative splicing of the TRPC3 ion channel calmodulin/IP3 receptor-binding domain in the hindbrain enhances cation flux.
Kim Y, Wong AC, Power JM, Tadros SF, Klugmann M, Moorhouse AJ, Bertrand PP, Housley GD. Kim Y, et al. J Neurosci. 2012 Aug 15;32(33):11414-23. doi: 10.1523/JNEUROSCI.6446-11.2012. J Neurosci. 2012. PMID: 22895723 Free PMC article. - TRPC3 Channel Activity and Viability of Purkinje Neurons can be Regulated by a Local Signalosome.
Aslam N, Alvi F. Aslam N, et al. Front Mol Biosci. 2022 Feb 21;9:818682. doi: 10.3389/fmolb.2022.818682. eCollection 2022. Front Mol Biosci. 2022. PMID: 35265671 Free PMC article. - Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors.
Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, Dusart I, Fagni L, Lambolez B, Levenes C. Ady V, et al. EMBO Rep. 2014 Jan;15(1):103-9. doi: 10.1002/embr.201337371. Epub 2013 Dec 15. EMBO Rep. 2014. PMID: 24357660 Free PMC article.
References
- Yuan J. X. J., Ward J. P. T., Song M. Y., Yuan J. X. J. (2010) Membrane Receptors, Channels and Transporters in Pulmonary Circulation (Yuan J. X. J., Ward J. P. T., eds) pp. 99–109, Humana Press Inc., Totowa, NJ
- Kiselyov K., van Rossum D. B., Patterson R. L., Gerald L. (2010) in Vitamins and Hormones (Litwack G., ed) pp. 197–213, Academic Press, New York - PubMed
- Glitsch M. D. (2010) Activation of native TRPC3 cation channels by phospholipase D. FASEB J. 24, 318–325 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources