The many roles of TOX in the immune system - PubMed (original) (raw)
Review
The many roles of TOX in the immune system
Parinaz Aliahmad et al. Curr Opin Immunol. 2012 Apr.
Abstract
TOX is a member of an evolutionarily conserved DNA-binding protein family and is expressed in several immune-relevant cell subsets. Here, we review the key role of TOX in regulating development of CD4 T cells, natural killer cells and lymphoid tissue inducer cells, the latter responsible for the generation of lymph nodes. Although the exact molecular mechanism of action of TOX remains to be elucidated, the role of TOX in establishment of gene programs in the thymus and the potential of TOX as a regulator of E protein activity are discussed.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
The TOX subfamily of HMG-box proteins. TOX contains an HMG-box DNA binding domain that is highly conserved in three additional proteins TOX2, TOX3, and TOX4. An adjacent lysine-rich region may serve as the nuclear localization signal (NLS) as well as influence the interaction with DNA. The N-terminal domains of these proteins show approximately 30–40% sequence identity and have transactivation activity, while the C-terminal domains differ greatly between family members.
Figure 2
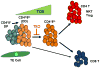
TOX is a key regulator of T cell development in the thymus. TCR signaling in DP thymocytes undergoing positive selection, through interaction with thymic epithelial (TE) cells, causes downregulation of CD4 and CD8 resulting in a double dull (DD) phenotype followed by re-expression of CD4 to yield CD4+CD8lo cells. The latter subpopulation of cells contains the immediate precursors of both CD4+CD8− (CD4SP) and CD4− CD8+ (CD8SP) thymocytes, although some CD8SP can derive directly from DD cells. TOX is induced by TCR signals early in positive selection, first detected in DD cells and further upregulated at the CD4+CD8lo stage, before returning to baseline levels in SP thymocytes. In the absence of TOX (TKO), the DD to CD4+CD8lo progression is severely inhibited, blocking development of CD4 T cells but with a more modest effect on development of CD8 T cells.
Figure 3
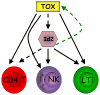
TOX is required for development of NK, LTi and CD4 T cells, possibly by modulating E protein activity. Both NK and LTi lineages share dependence on expression of Id2 as well as TOX. TKO NK cells (and thymocytes) have reduced expression of Id2, suggesting that TOX may be an upstream regulator of this E protein inhibitor. However, it is likely that TOX also regulates other events required for development of these cell lineages, as reconstitution of Id2 did not rescue NK cell development in TKO bone marrow precursors. Id2 is also expressed in the thymus, but its deletion does not cause a block in T cell development possibly due to compensation by Id3 [36,37]. However, TKO thymocytes express lower levels of Id2, and there is failure to upregulate some genes that are repressed by E proteins [13,21]. Whether this is causally linked remains to be proven. Thus, the role of Id2 during thymocyte development may need to be revisited. Interestingly, as E protein activity has also been shown to have a negative effect on TOX expression [24], a feed forward regulatory circuit might enforce TOX expression in these different cell types. (In this Figure, dashed lines refer to hypotheticals).
Similar articles
- The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches.
Eberl G, Littman DR. Eberl G, et al. Immunol Rev. 2003 Oct;195:81-90. doi: 10.1034/j.1600-065x.2003.00074.x. Immunol Rev. 2003. PMID: 12969312 Review. - Affecting the effectors: a kick in the gut?
Johansson C, Kelsall BL. Johansson C, et al. Nat Immunol. 2005 Jul;6(7):644-6. doi: 10.1038/ni0705-644. Nat Immunol. 2005. PMID: 15970934 No abstract available. - Induction of IgA B cell differentiation of bone marrow-derived B cells by Peyer's patch autoreactive helper T cells.
Kihira T, Kawanishi H. Kihira T, et al. Immunol Invest. 1995 Aug;24(5):701-11. doi: 10.3109/08820139509060699. Immunol Invest. 1995. PMID: 8543335 - Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches.
Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Tsuji M, et al. Science. 2009 Mar 13;323(5920):1488-92. doi: 10.1126/science.1169152. Science. 2009. PMID: 19286559 - Specific lymphocyte-endothelial cell interactions regulate migration into lymph nodes, Peyer's patches, and skin.
Chin YH, Falanga V, Streilein JW, Sackstein R. Chin YH, et al. Reg Immunol. 1988 Jul-Aug;1(1):78-83. Reg Immunol. 1988. PMID: 3079311 Review.
Cited by
- Transcriptional rewiring in CD8+ T cells: implications for CAR-T cell therapy against solid tumours.
Srinivasan S, Armitage J, Nilsson J, Waithman J. Srinivasan S, et al. Front Immunol. 2024 Sep 27;15:1412731. doi: 10.3389/fimmu.2024.1412731. eCollection 2024. Front Immunol. 2024. PMID: 39399500 Free PMC article. Review. - TOX2 nuclear-cytosol translocation is linked to leukemogenesis of acute T-cell leukemia by repressing TIM3 transcription.
Li A, Zhang J, Zhan L, Liu X, Zeng X, Zhu Q, Wang Z, Li J. Li A, et al. Cell Death Differ. 2024 Nov;31(11):1506-1518. doi: 10.1038/s41418-024-01352-z. Epub 2024 Jul 30. Cell Death Differ. 2024. PMID: 39080376 Free PMC article. - The TOX-RAGE axis mediates inflammatory activation and lung injury in severe pulmonary infectious diseases.
Kim H, Park HH, Kim HN, Seo D, Hong KS, Jang JG, Seo EU, Kim IY, Jeon SY, Son B, Cho SW, Kim W, Ahn JH, Lee W. Kim H, et al. Proc Natl Acad Sci U S A. 2024 Jun 25;121(26):e2319322121. doi: 10.1073/pnas.2319322121. Epub 2024 Jun 20. Proc Natl Acad Sci U S A. 2024. PMID: 38900789 Free PMC article. - The nexus of dynamic T cell states and immune checkpoint blockade therapy in the periphery and tumor microenvironment.
Luo H, Wang W, Mai J, Yin R, Cai X, Li Q. Luo H, et al. Front Immunol. 2023 Oct 10;14:1267918. doi: 10.3389/fimmu.2023.1267918. eCollection 2023. Front Immunol. 2023. PMID: 37881432 Free PMC article. Review. - Unusual herpetic reactivation in a young female following botox injection: a case report study.
Amini-Salehi E, Eslami N, Tamimi A, Sedighi N, Moghdam SS, Yaghubi-Kalurazi T, Hassanipour S, Joukar F, Mansour-Ghanaei F, Eftekhari H. Amini-Salehi E, et al. BMC Infect Dis. 2023 Oct 2;23(1):647. doi: 10.1186/s12879-023-08514-3. BMC Infect Dis. 2023. PMID: 37784014 Free PMC article.
References
- Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat Immunol. 2002;3:272–280. - PubMed
- Kajitani T, Mizutani T, Yamada K, Yazawa T, Sekiguchi T, Yoshino M, Kawata H, Miyamoto K. Cloning and characterization of granulosa cell high-mobility group (HMG)-box protein-1, a novel HMG-box transcriptional regulator strongly expressed in rat ovarian granulosa cells. Endocrinology. 2004;145:2307–2318. - PubMed
- Dittmer S, Kovacs Z, Yuan SH, Siszler G, Kogl M, Summer H, Geerts A, Golz S, Shioda T, Methner A. TOX3 is a neuronal survival factor that induces transcription depending on the presence of CITED1 or phosphorylated CREB in the transcriptionally active complex. J Cell Sci. 2011;124:252–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials