Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers - PubMed (original) (raw)
Review
Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers
Laura M Ensign et al. Adv Drug Deliv Rev. 2012.
Abstract
Oral delivery is the most common method for drug administration. However, poor solubility, stability, and bioavailability of many drugs make achieving therapeutic levels via the gastrointestinal (GI) tract challenging. Drug delivery must overcome numerous hurdles, including the acidic gastric environment and the continuous secretion of mucus that protects the GI tract. Nanoparticle drug carriers that can shield drugs from degradation and deliver them to intended sites within the GI tract may enable more efficient and sustained drug delivery. However, the rapid secretion and shedding of GI tract mucus can significantly limit the effectiveness of nanoparticle drug delivery systems. Many types of nanoparticles are efficiently trapped in and rapidly removed by mucus, making controlled release in the GI tract difficult. This review addresses the protective barrier properties of mucus secretions, how mucus affects the fate of orally administered nanoparticles, and recent developments in nanoparticles engineered to penetrate the mucus barrier.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
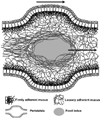
Figure 1
Schematic figure showing the thicknesses of the loosely adherent and firmly adherent mucus layers in vivo in the rat gastrointestinal tract. The loosely adherent mucus layer is removable by careful suction, whereas the firmly adherent mucus layer is not. The table presents values for mucus thickness as means ± SE for each group. Figure obtained from [18].
Figure 2
Summary schematic illustrating the fate of an ingested food bolus propelled through the GI tract via peristaltic contractions. Mucins in the loosely adherent layer adhere to the food, wrapping it in a ‘blanket’ of mucus. The shear thinning properties of the secreted mucins allow the bolus to pass without perturbing the firmly adherent layer and the epithelium. Enzymes and emulsifying lipids that can pass through the mucus will begin to digest the food, extracting nutrients. Water is continuously removed from any undigested material as it passes through the small intestine and colon.
Figure 3
Schematic model depicting the possible role of an extracellular lining of zwitterionic phospholipids in generating the hydrophobic barrier of the stomach to luminal acid. Figure adapted from [30].
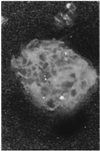
Figure 4
Agglomerated Eudragit® particles coated with a mucus plug collected immediately following discharge from the proximal jejunum of the perfused rat colon (magnification ×60). Figure obtained from [40].
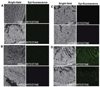
Figure 5
Qualitative enhanced green fluorescent protein (GFP) expression in the small and large intestinal tract of male Wistar rats cryosections after oral administrate of saline (A), naked GFP plasmid (B), gelatin nanoparticles encapsulating GFP plasmid (C) and the NiMOS encapsulating GFP plasmid (D). The bright-field and epifluorescence images of the tissue cryosections were obtained from small intestine and large intestine of rats after 5 days following a single 100 µg dose of plasmid administered orally in the control and NiMOS formulations. The letter “L” denotes the luminal side of the small and large intestine. Figure obtained from [79].
Figure 6
Snapshot of the concentration field during the displacement of a more viscous fluid (dark) by a fully-miscible, less viscous fluid (light). Figure obtained from [128].
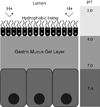
Figure 7
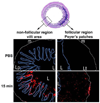
Distribution of the mucus gel layer in front of the Peyer’s patch in (A) a control group and (B) the N-acetylcysteine (NAC) group. Periodic acid-Schiff staining shows a uniform, continuous layer of mucus gel in front of the Peyer’s patch that is then completely lacking after NAC treatment. L, lumen; GC, germinal center. Magnification, ×25. Figure adapted from [100].
Figure 8
Simultaneous uptake of equal numbers of CTB-coated (red) and control microparticles (green) into rabbit Peyer’s patch domes after 1 h exposure. Fluorescence microscopy of a representative cryostat section shows that both types of particles were taken up into the dome, but not into adjacent villi. Scale bar, 100 µm. Figure obtained from [2].
Figure 9
Fluorescent micrographs obtained from transversal cryosections of mice ileum intestine. In vivo intestinal ligated loop was incubated with 10 mg/mL of CellTrace BODIPY nanoparticles (NPs) diluted in PBS for 15, 30, 45, and 60 min (only showing 15 mins, trends similar across all time points). Micrographs were obtained from villus and Peyer’s patch (PP) areas. The letter L indicates the intestinal lumen, the muscularis mucosa is underscored with a white line (m), and the epithelial barrier is indicated by a blue line (E), around the Lamina propia (Lp in villus area) or the lymphoid tissue (Lt in PP area). Original magnification ×10. Figure adapted from [106].
Figure 10
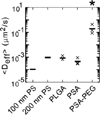
Geometric ensemble effective diffusivity (
) at a time scale of 1 sec for polystyrene (PS), poly(lactic-co-glycolic acid) (PLGA), poly(sebacic acid) (PSA), and poly(sebacic acid)-co-poly(ethylene glycol) (PSA-PEG) in CVM. × denotes individual sample values (n = 3); – denotes the average. Figure adapted from [8].
Figure 11
A summary schematic illustrating the fate of mucus penetrating particles (MPP) and conventional mucoadhesive particles (CP) administered to the GI mucosal surface. MPP readily penetrate the loosely adherent mucus layer and enter the firmly adherent mucus layer. In contrast, CP are immobilized in the loosely adherent layer. Because MPP can enter the firmly adherent layer and thus are in closer proximity to cells, cells will be exposed to a greater dose of drug released from MPP compared to drug released from CP. As the loosely adherent layer is cleared, CP are removed, whereas MPP are retained longer within the firmly adherent layer and continue to release drugs to cells. Figure adapted from [5].
Similar articles
- Oral drug delivery with nanoparticles into the gastrointestinal mucosa.
Liu J, Leng P, Liu Y. Liu J, et al. Fundam Clin Pharmacol. 2021 Feb;35(1):86-96. doi: 10.1111/fcp.12594. Epub 2020 Aug 25. Fundam Clin Pharmacol. 2021. PMID: 32749731 Review. - Nanoparticles for oral delivery: Design, evaluation and state-of-the-art.
Date AA, Hanes J, Ensign LM. Date AA, et al. J Control Release. 2016 Oct 28;240:504-526. doi: 10.1016/j.jconrel.2016.06.016. Epub 2016 Jun 9. J Control Release. 2016. PMID: 27292178 Free PMC article. Review. - Nanocarriers protecting toward an intestinal pre-uptake metabolism.
Suchaoin W, Bernkop-Schnürch A. Suchaoin W, et al. Nanomedicine (Lond). 2017 Feb;12(3):255-269. doi: 10.2217/nnm-2016-0331. Epub 2017 Jan 17. Nanomedicine (Lond). 2017. PMID: 28093952 Review. - Polymeric nanoparticle drug delivery technologies for oral delivery applications.
Pridgen EM, Alexis F, Farokhzad OC. Pridgen EM, et al. Expert Opin Drug Deliv. 2015;12(9):1459-73. doi: 10.1517/17425247.2015.1018175. Epub 2015 Mar 26. Expert Opin Drug Deliv. 2015. PMID: 25813361 Free PMC article. Review. - Mechanisms of Nanoparticle Transport across Intestinal Tissue: An Oral Delivery Perspective.
Ejazi SA, Louisthelmy R, Maisel K. Ejazi SA, et al. ACS Nano. 2023 Jul 25;17(14):13044-13061. doi: 10.1021/acsnano.3c02403. Epub 2023 Jul 6. ACS Nano. 2023. PMID: 37410891 Review.
Cited by
- Poly(amido amine) dendrimers in oral delivery.
Yellepeddi VK, Ghandehari H. Yellepeddi VK, et al. Tissue Barriers. 2016 Apr 6;4(2):e1173773. doi: 10.1080/21688370.2016.1173773. eCollection 2016 Apr-Jun. Tissue Barriers. 2016. PMID: 27358755 Free PMC article. Review. - Enhanced oral bioavailability of acetylpuerarin by poly(lactide-co-glycolide) nanoparticles optimized using uniform design combined with response surface methodology.
Sun D, Xue A, Zhang B, Xue X, Zhang J, Liu W. Sun D, et al. Drug Des Devel Ther. 2016 Jun 21;10:2029-39. doi: 10.2147/DDDT.S108185. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27382256 Free PMC article. - Recent Review on Biological Barriers and Host-Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies.
Deshmukh R, Sethi P, Singh B, Shiekmydeen J, Salave S, Patel RJ, Ali N, Rashid S, Elossaily GM, Kumar A. Deshmukh R, et al. Pharmaceutics. 2024 Aug 16;16(8):1076. doi: 10.3390/pharmaceutics16081076. Pharmaceutics. 2024. PMID: 39204421 Free PMC article. Review. - Colloidal aggregation: from screening nuisance to formulation nuance.
Ganesh AN, Donders EN, Shoichet BK, Shoichet MS. Ganesh AN, et al. Nano Today. 2018 Apr;19:188-200. doi: 10.1016/j.nantod.2018.02.011. Epub 2018 Mar 10. Nano Today. 2018. PMID: 30250495 Free PMC article. - Extremophile-based biohybrid micromotors for biomedical operations in harsh acidic environments.
Zhang F, Li Z, Duan Y, Luan H, Yin L, Guo Z, Chen C, Xu M, Gao W, Fang RH, Zhang L, Wang J. Zhang F, et al. Sci Adv. 2022 Dec 23;8(51):eade6455. doi: 10.1126/sciadv.ade6455. Epub 2022 Dec 23. Sci Adv. 2022. PMID: 36563149 Free PMC article.
References
- Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Cl Ga. 2008;22:391–409. - PubMed
- Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: Implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. - PMC - PubMed
- Plapied L, Duhem N, des Rieux A, Preat V. Fate of polymeric nanocarriers for oral drug delivery. Curr Opin Colloid In. 2011;16:228–237.
- Cone RA. Barrier properties of mucus. Advanced Drug Delivery Reviews. 2009;61:75–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R33 AI079740/AI/NIAID NIH HHS/United States
- R01 HD062844-01/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 5R01HD062844/HD/NICHD NIH HHS/United States
- 5R21AI094519/AI/NIAID NIH HHS/United States
- R21 AI094519/AI/NIAID NIH HHS/United States
- R01 HD062844/HD/NICHD NIH HHS/United States
- R33 AI094519/AI/NIAID NIH HHS/United States
- R33 AI079740-03/AI/NIAID NIH HHS/United States
- R21 AI094519-01/AI/NIAID NIH HHS/United States
- 5R33AI079740/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources