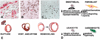Regulation of the inflammatory response in cardiac repair - PubMed (original) (raw)
Review
Regulation of the inflammatory response in cardiac repair
Nikolaos G Frangogiannis. Circ Res. 2012.
Abstract
Myocardial necrosis triggers an inflammatory reaction that clears the wound from dead cells and matrix debris, while activating reparative pathways necessary for scar formation. A growing body of evidence suggests that accentuation, prolongation, or expansion of the postinfarction inflammatory response results in worse remodeling and dysfunction following myocardial infarction. This review manuscript discusses the cellular effectors and endogenous molecular signals implicated in suppression and containment of the inflammatory response in the infarcted heart. Clearance of apoptotic neutrophils, recruitment of inhibitory monocyte subsets and regulatory T cells, macrophage differentiation and pericyte/endothelial interactions may play an active role in restraining postinfarction inflammation. Multiple molecular signals may be involved in suppressing the inflammatory cascade. Negative regulation of toll-like receptor signaling, downmodulation of cytokine responses, and termination of chemokine signals may be mediated through the concerted action of multiple suppressive pathways that prevent extension of injury and protect from adverse remodeling. Expression of soluble endogenous antagonists, decoy receptors, and posttranslational processing of bioactive molecules may limit cytokine and chemokine actions. Interleukin-10, members of the transforming growth factor-β family, and proresolving lipid mediators (such as lipoxins, resolvins, and protectins) may suppress proinflammatory signaling. In human patients with myocardial infarction, defective suppression, and impaired resolution of inflammation may be important mechanisms in the pathogenesis of remodeling and in progression to heart failure. Understanding of inhibitory and proresolving signals in the infarcted heart and identification of patients with uncontrolled postinfarction inflammation and defective cardiac repair is needed to design novel therapeutic strategies.
Figures
Figure 1. The consequences of unrestrained inflammation in the infarcted heart
Repair of the infarcted heart is dependent on timely suppression of the post-infarction inflammatory response and on resolution of the inflammatory infiltrate. A-C: Histopathologic illustration of induction, suppression and resolution of the post-infarction inflammatory response in a canine model. Dual immunohistochemical staining combines staining for Mac387 (red), a myeloid cell marker that labels neutrophils and monocytes (but not mature macrophages), serving as a measure of active leukocyte recruitment in inflamed tissues and staining for PM-2K, a mature macrophage marker that is not expressed in newly-recruited monocytes. After 24h of reperfusion abundant newly-recruited myeloid cells are identified in the infarct; however; their number decreases significantly after 7 days reflecting suppression of active inflammatory cell recruitment. At this stage, PM-2K+ mature macrophages are the predominant leukocyte type in the infarct. After 28 days of reperfusion resolution of the leukocyte infiltrate is noted. D: : Expected effects of uncontrolled and excessive inflammation on the cell types involved in cardiac repair. Prolongation or accentuation of the inflammatory reaction may result in increased leukocyte activation, enhanced adhesiveness and permeability of endothelial cells, acquisition of a matrix-degrading phenotype by fibroblasts and cardiomyocyte apoptosis. E: Impact of the cellular effects of overactive, prolonged or poorly-contained inflammation on the infarcted heart. Cardiac rupture, formation of ventricular aneurysms, increased dysfunction and adverse dilative remodeling could result from defective regulation of the post-infarction inflammatory response.
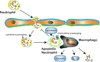
Figure 2. The role of neutrophil clearance in suppression of the inflammatory response
Abundant neutrophils infiltrate the infarcted myocardium. Neutrophils are short-lived cells that undergo apoptosis; dying neutrophils may contribute to repression of the post-infarction inflammatory response through several distinct mechanisms. First apoptotic neutrophils may release lactoferrin, an inhibitor of granulocyte transmigration. Second, during clearance of apoptotic neutrophils, macrophages secrete large amounts of anti-inflammatory and pro-resolving mediators including IL-10, TGF-β and lipoxins. Third, expression of decoy cytokine receptors by neutrophils may promote cytokine scavenging. Increased expression of chemokine receptors (such as CCR5) in apoptotic neutrophils may serve as a molecular trap for chemokines terminating their action.
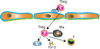
Figure 3. Regulatory T cells (Tregs) may suppress inflammation following myocardial infarction
Molecular interactions involving yet unidentified chemokines presented by endothelial cells and their corresponding chemokine receptors mediate infiltration of the infarcted myocardium with Tregs. Tregs may suppress inflammation by secreting inhibitory signals such as IL-10 and TGF-β and through contact-mediated actions. T, effector T cell; Ma, macrophage.
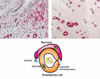
Figure 4. Vascular maturation through acquisition of a mural cell coat may contribute to inhibition of inflammation following myocardial infarction
Upper panel: Dual immunohistochemical staining for the endothelial cell marker CD31 (black) and α-smooth muscle actin (red) to detect vascular mural cells illustrates the maturation process in canine infarct neovessels. After 7 days of reperfusion the infarct contains abundant microvessels; the majority lacks coverage with mural cells. In the mature scar after 28 days of reperfusion, a large number of coated vessels is noted, whereas most capillaries have regressed. Acquisition of a pericyte coat involves PDGF-BB/PDGFR-β interactions and contributes to stabilization of the scar reducing the inflammatory and angiogenic activity of endothelial cells.
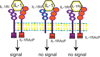
Figure 5. Negative regulation of IL-1-mediated inflammation
Termination of IL-1-driven inflammation may involve a competitive inhibitor (IL-1Ra) and a decoy receptor (IL-1RII). IL-1RAcP, IL-1 receptor accessory protein..
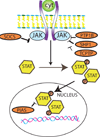
Figure 6. Negative regulation of cytokine signaling
Intracellular signals such as the SOCS proteins and several phosphatases interfere with JAK-STAT signaling inhibiting cytokine responses (see text).
Similar articles
- The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis.
Prabhu SD, Frangogiannis NG. Prabhu SD, et al. Circ Res. 2016 Jun 24;119(1):91-112. doi: 10.1161/CIRCRESAHA.116.303577. Circ Res. 2016. PMID: 27340270 Free PMC article. Review. - The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities.
Frangogiannis NG. Frangogiannis NG. J Cardiovasc Pharmacol. 2014 Mar;63(3):185-95. doi: 10.1097/FJC.0000000000000003. J Cardiovasc Pharmacol. 2014. PMID: 24072174 Free PMC article. Review. - Targeting inflammatory pathways in myocardial infarction.
Christia P, Frangogiannis NG. Christia P, et al. Eur J Clin Invest. 2013 Sep;43(9):986-95. doi: 10.1111/eci.12118. Epub 2013 Jun 17. Eur J Clin Invest. 2013. PMID: 23772948 Free PMC article. Review. - The immune system and cardiac repair.
Frangogiannis NG. Frangogiannis NG. Pharmacol Res. 2008 Aug;58(2):88-111. doi: 10.1016/j.phrs.2008.06.007. Epub 2008 Jun 24. Pharmacol Res. 2008. PMID: 18620057 Free PMC article. Review. - The inflammatory response in myocardial injury, repair, and remodelling.
Frangogiannis NG. Frangogiannis NG. Nat Rev Cardiol. 2014 May;11(5):255-65. doi: 10.1038/nrcardio.2014.28. Epub 2014 Mar 25. Nat Rev Cardiol. 2014. PMID: 24663091 Free PMC article. Review.
Cited by
- Yixin-Shu Capsules Ameliorated Ischemia-Induced Heart Failure by Restoring Trx2 and Inhibiting JNK/p38 Activation.
Xiang C, Zhang F, Gao J, Guo F, Zhang M, Zhou R, Wei J, Wang P, Zhang Y, Zhang J, Yang H. Xiang C, et al. Oxid Med Cell Longev. 2021 Feb 16;2021:8049079. doi: 10.1155/2021/8049079. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33643519 Free PMC article. - Neuraminidase-1 promotes heart failure after ischemia/reperfusion injury by affecting cardiomyocytes and invading monocytes/macrophages.
Heimerl M, Sieve I, Ricke-Hoch M, Erschow S, Battmer K, Scherr M, Hilfiker-Kleiner D. Heimerl M, et al. Basic Res Cardiol. 2020 Sep 25;115(6):62. doi: 10.1007/s00395-020-00821-z. Basic Res Cardiol. 2020. PMID: 32975669 Free PMC article. - Extracellular vesicles derived from Krüppel-Like Factor 2-overexpressing endothelial cells attenuate myocardial ischemia-reperfusion injury by preventing Ly6Chigh monocyte recruitment.
Qiao S, Zhang W, Yin Y, Wei Z, Chen F, Zhao J, Sun X, Mu D, Xie J, Xu B. Qiao S, et al. Theranostics. 2020 Sep 18;10(25):11562-11579. doi: 10.7150/thno.45459. eCollection 2020. Theranostics. 2020. PMID: 33052233 Free PMC article. - The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis.
Prabhu SD, Frangogiannis NG. Prabhu SD, et al. Circ Res. 2016 Jun 24;119(1):91-112. doi: 10.1161/CIRCRESAHA.116.303577. Circ Res. 2016. PMID: 27340270 Free PMC article. Review. - Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action.
Jung I, Kim H, Moon S, Lee H, Kim B. Jung I, et al. Antioxidants (Basel). 2020 Sep 13;9(9):857. doi: 10.3390/antiox9090857. Antioxidants (Basel). 2020. PMID: 32933217 Free PMC article. Review.
References
- Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. - PubMed
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. - PubMed
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL076246/HL/NHLBI NIH HHS/United States
- R01 HL085440/HL/NHLBI NIH HHS/United States
- R01 HL-85440/HL/NHLBI NIH HHS/United States
- R01 HL-76246/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical