Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys - PubMed (original) (raw)
Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys
Susan H McKiernan et al. Exp Gerontol. 2012 Mar.
Abstract
We have previously shown that a 30% reduced calorie intake diet delayed the onset of muscle mass loss in adult monkeys between ~16 and ~22 years of age and prevented multiple cellular phenotypes of aging. In the present study we show the impact of long term (~17 years) calorie restriction (CR) on muscle aging in very old monkeys (27-33 yrs) compared to age-matched Control monkeys fed ad libitum, and describe these data in the context of the whole longitudinal study. Muscle mass was preserved in very old calorie restricted (CR) monkeys compared to age-matched Controls. Immunohistochemical analysis revealed an age-associated increase in the proportion of Type I fibers in the VL from Control animals that was prevented with CR. The cross sectional area (CSA) of Type II fibers was reduced in old CR animals compared to earlier time points (16-22 years of age); however, the total loss in CSA was only 15% in CR animals compared to 36% in old Controls at ~27 years of age. Atrophy was not detected in Type I fibers from either group. Notably, Type I fiber CSA was ~1.6 fold greater in VL from CR animals compared to Control animals at ~27 years of age. The frequency of VL muscle fibers with defects in mitochondrial electron transport system enzymes (ETS(ab)), the absence of cytochrome c oxidase and hyper-reactive succinate dehydrogenase, were identical between Control and CR. We describe changes in ETS(ab) fiber CSA and determined that CR fibers respond differently to the challenge of mitochondrial deficiency. Fiber counts of intact rectus femoris muscles revealed that muscle fiber density was preserved in old CR animals. We suggest that muscle fibers from CR animals are better poised to endure and adapt to changes in muscle mass than those of Control animals.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1
Body and VL muscle composition of aged (~27 y) rhesus monkeys. Vertical bars represent the mean values for control (black) and restricted (white) and small square boxes represent individual monkeys: a). body weight, p=0.035; b). body fat, p = 0.038; c). percent upper leg muscle mass expressed as a proportion of estimated upper leg mass at ~27 years of age over maximum upper leg muscle mass observed, p = 0.015); d). percentage of Type I muscle fibers, p = 0.02; e). Type I fiber cross-sectional area (CSA), p < 0.01 and f). Type II fiber CSA, p = 0.70)
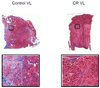
Figure 2
Masson’s trichrome stain for collagen on aged rhesus monkeys’ VL. Muscle stains red, collagen stains blue. Scanned images (2×) and close ups (20×).
Figure 3
Histogram of the frequency of normal and ETSab abnormal muscle fibers based on minimum cross-sectional area (CSA) ratio (the proportion between the minimum CSA of a fiber within the ETSab region and the mean CSA of the normal region of the fiber). Significant differences in CSA ratio distributions were observed between Control ETSab fibers and CR ETSab fibers (p = 0.022) and between CR normal fibers and CR ETSab fibers (p < 0.001).
Figure 4
Longitudinal analysis of Control and CR rhesus monkey VL muscle after an average of 17 years in the study. a). Upper leg muscle mass / maximum upper leg muscle mass. Both Control (n=9) and CR (n=11) undergo significant declines with age (p < 0.001), CR were significantly higher overall (p = 0.04) and Controls have a significantly sharper rate of loss compared to Controls (p < 0.001); b). Type II fiber CSA. Significant reduction in Type II fiber CSA in Control VL (n=8) occurs between ~16- and ~22-years of age, CR VL (n=10) Type II fiber CSA is relatively constant between the same years and begins to decline at ~27 years of age, Type II fibers from Controls have a significantly different trend for age-dependent atrophy compared to CR (p = 0.04); c). VL Type I fiber CSA significantly increased with age in both Control (n=8) and CR (n=10; p < 0.001), Type I fiber CSA of CR monkeys was significantly higher than Control (p < 0.001) and the rate of increase was significantly higher in CR (p < 0.001) and d). Percentage of ETSab fibers in 2000µm of VL tissue. A significant increase with age was observed in both Control (n=8) and CR (n=11). Graphs represent data accumulated between years 6 and 19 of the CR study with animals between the ages of ~16y and ~27y.
Similar articles
- Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle.
McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, Weindruch R. McKiernan SH, et al. Exp Gerontol. 2011 Jan;46(1):23-9. doi: 10.1016/j.exger.2010.09.011. Epub 2010 Sep 29. Exp Gerontol. 2011. PMID: 20883771 Free PMC article. - Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle.
Bua E, McKiernan SH, Aiken JM. Bua E, et al. FASEB J. 2004 Mar;18(3):582-4. doi: 10.1096/fj.03-0668fje. Epub 2004 Jan 20. FASEB J. 2004. PMID: 14734641 - Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction.
Phillips T, Leeuwenburgh C. Phillips T, et al. FASEB J. 2005 Apr;19(6):668-70. doi: 10.1096/fj.04-2870fje. Epub 2005 Jan 21. FASEB J. 2005. PMID: 15665035 - Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice.
Lee CM, Aspnes LE, Chung SS, Weindruch R, Aiken JM. Lee CM, et al. Ann N Y Acad Sci. 1998 Nov 20;854:182-91. doi: 10.1111/j.1749-6632.1998.tb09901.x. Ann N Y Acad Sci. 1998. PMID: 9928429 Review. - Caloric restriction: implications for sarcopenia and potential mechanisms.
Xie WQ, Xiao WF, Tang K, Wu YX, Hu PW, Li YS, Duan Y, Lv S. Xie WQ, et al. Aging (Albany NY). 2020 Nov 21;12(23):24441-24452. doi: 10.18632/aging.103987. Epub 2020 Nov 21. Aging (Albany NY). 2020. PMID: 33226962 Free PMC article. Review.
Cited by
- Age-dependent effects of caloric restriction on mTOR and ubiquitin-proteasome pathways in skeletal muscles.
Chen CN, Liao YH, Tsai SC, Thompson LV. Chen CN, et al. Geroscience. 2019 Dec;41(6):871-880. doi: 10.1007/s11357-019-00109-8. Epub 2019 Nov 1. Geroscience. 2019. PMID: 31676964 Free PMC article. - Regulation of metabolic health and aging by nutrient-sensitive signaling pathways.
Cummings NE, Lamming DW. Cummings NE, et al. Mol Cell Endocrinol. 2017 Nov 5;455:13-22. doi: 10.1016/j.mce.2016.11.014. Epub 2016 Nov 22. Mol Cell Endocrinol. 2017. PMID: 27884780 Free PMC article. Review. - Calorie Restriction and Aging in Humans.
Flanagan EW, Most J, Mey JT, Redman LM. Flanagan EW, et al. Annu Rev Nutr. 2020 Sep 23;40:105-133. doi: 10.1146/annurev-nutr-122319-034601. Epub 2020 Jun 19. Annu Rev Nutr. 2020. PMID: 32559388 Free PMC article. - Sirt1: def-eating senescence?
Fusco S, Maulucci G, Pani G. Fusco S, et al. Cell Cycle. 2012 Nov 15;11(22):4135-46. doi: 10.4161/cc.22074. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22983125 Free PMC article. Review. - The Impact of Aging, Calorie Restriction and Dietary Fat on Autophagy Markers and Mitochondrial Ultrastructure and Dynamics in Mouse Skeletal Muscle.
Gutiérrez-Casado E, Khraiwesh H, López-Domínguez JA, Montero-Guisado J, López-Lluch G, Navas P, de Cabo R, Ramsey JJ, González-Reyes JA, Villalba JM. Gutiérrez-Casado E, et al. J Gerontol A Biol Sci Med Sci. 2019 May 16;74(6):760-769. doi: 10.1093/gerona/gly161. J Gerontol A Biol Sci Med Sci. 2019. PMID: 30010806 Free PMC article.
References
- Aiken J, Bua E, Cao Z, Lopez M, Wanagat J, McKenzie D, McKiernan S. Mitochondrial DNA deletion mutations and sarcopenia. Ann NY Acad Sci. 2002;959:412–423. - PubMed
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. - PubMed
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging. 1998;10:83–92. - PubMed
Publication types
MeSH terms
Grants and funding
- RR020141-01/RR/NCRR NIH HHS/United States
- R01 AG037000/AG/NIA NIH HHS/United States
- R01 AG040178/AG/NIA NIH HHS/United States
- P01 AG011915/AG/NIA NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- P01 AG-11915/AG/NIA NIH HHS/United States
- C06 RR020141/RR/NCRR NIH HHS/United States
- C06 RR015459/RR/NCRR NIH HHS/United States
- P51 RR000167/RR/NCRR NIH HHS/United States
- RR15459-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical