D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation - PubMed (original) (raw)
. 2012 Jun;166(4):1344-56.
doi: 10.1111/j.1476-5381.2012.01834.x.
Carla Iacobini, Carlo Ricci, Angela Scipioni, Claudia Blasetti Fantauzzi, Andrea Giaccari, Enrica Salomone, Renato Canevotti, Annunziata Lapolla, Marica Orioli, Giancarlo Aldini, Giuseppe Pugliese
Affiliations
- PMID: 22229552
- PMCID: PMC3417451
- DOI: 10.1111/j.1476-5381.2012.01834.x
D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation
Stefano Menini et al. Br J Pharmacol. 2012 Jun.
Abstract
Background and purpose: Lipoxidation-derived reactive carbonyl species (RCS) such as 4-hydroxy-2-nonenal (HNE) react with proteins to form advanced lipoxidation end products (ALEs), which have been implicated in both atherosclerosis and renal disease. L-carnosine acts as an endogenous HNE scavenger, but it is rapidly inactivated by carnosinase. This study aimed at assessing the effect of the carnosinase-resistant, D-carnosine, on HNE-induced cellular injury and of its bioavailable prodrug D-carnosine octylester on experimental atherosclerosis and renal disease.
Experimental approach: Vascular smooth muscle cells (VSMCs) were exposed to HNE or H₂O₂ plus D-carnosine. ApoE null mice fed a Western, pro-atherogenic diet were treated with D-carnosine octylester for 12 weeks.
Key results: In vitro, D-carnosine attenuated the effect of HNE, but not of H₂O₂, on VSMCs. In vivo, D-carnosine octylester-treated mice showed reduced lesion area and a more stable plaque phenotype compared with untreated animals, with reduced foam cell accumulation, inflammation and apoptosis and increased clearance of apoptotic bodies and collagen deposition, resulting in decreased necrotic core formation. Likewise, renal lesions were attenuated in D-carnosine octylester-treated versus untreated mice, with lower inflammation, apoptosis and fibrosis. This was associated with increased urinary levels of HNE-carnosine adducts and reduced protein carbonylation, circulating and tissue ALEs, expression of receptors for these products, and systemic and tissue oxidative stress.
Conclusions and implications: These data indicate RCS quenching with a D-carnosine ester was highly effective in attenuating experimental atherosclerosis and renal disease by reducing carbonyl stress and inflammation and that this may represent a promising therapeutic strategy in humans.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
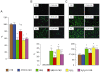
Figure 1
In vitro studies. Quantification of cell viability (panel A), Annexin-V-FLUOS fluorescence from representative monolayers and quantification of apoptosis rate (panel B) and DCF fluorescence from representative monolayers and quantification of ROS levels (panel C) in VSMCs incubated with saline CTR, HNE (10 µM for 4 h) or H2O2 (300 µM for 1 h), in the presence or absence of D-carnosine 20 mM. *P < 0.001 versus CTR monolayers; †P < 0.001 or ‡P < 0.01 versus monolayers incubated with HNE without D-carnosine. CTR = control.
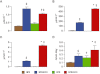
Figure 2
Carnosine levels. Plasma (panel A) and urinary (panel B) total carnosine, urinary D-carnosine octylester (panel C) and urine CAR-HNE (panel D) levels, in untreated and D-carnosine octylester-treated ApoE null mice fed a NFD or a HFD (mean ± SD; n_= 4 per group). *P < 0.001 or †_P < 0.05 versus NFD-fed mice; ‡P < 0.001 or §P < 0.01 versus untreated mice. DCO = D-carnosine octylester.
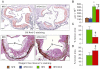
Figure 3
Aortic lesions. Oil Red O and Weigert-Van Gieson's staining of aortic sinus from representative untreated and D-carnosine octylester-treated ApoE null mice fed a HFD (panel A); and quantification of lesion area (panel B), necrotic core (panel C) and collagen content (panel D) in untreated and D-carnosine octylester-treated ApoE null mice fed a NFD (only for lesion area) or a HFD (mean ± SD; n_= 10 per group); scale bar = 100 µm. *P < 0.001 versus NFD-fed mice; †_P < 0.001 or ‡P < 0.01 versus untreated mice. L = lumen; M = macrophages; NC = necrotic core; C = collagen; DCO = D-carnosine octylester.
Figure 4
Aortic apoptosis, inflammation and VSMCs. Immunohistochemistry of aortic sinus from representative untreated and D-carnosine octylester-treated ApoE null mice fed a HFD and quantification of expression (mean ± SD; n_= 5 per group) for active caspase-3 (panel A), F4/80 (panel B), CXCR3 (panel C) and α-SMA (panel D) in untreated and D-carnosine octylester-treated ApoE null mice fed a HFD; scale bar = 50 µm. *P < 0.001 or †_P < 0.01 versus untreated mice. CXCR3 = chemokine receptor; α-SMA =α- smooth muscle actin; L = lumen; NC = necrotic core; TM = tunica media; FC = fibrous cap; DCO = D-carnosine octylester.
Figure 5
Aortic ALE levels and oxidative stress. Immunohistochemistry of aortic sinus from representative untreated and D-carnosine octylester-treated ApoE null mice fed a HFD and quantification of expression (mean ± SD; n_= 5 per group) for HNE adducts (panel A), oxLDLs (panel B) and nitrotyrosine (panel C); scale bar = 100 µm. *P < 0.001 or †_P < 0.01 versus the untreated mice. L = lumen; DCO = D-carnosine octylester.
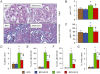
Figure 6
Kidney lesions. PAS and Masson's trichrome staining of kidneys from representative untreated and D-carnosine octylester-treated ApoE null mice fed a HFD (panel A; *= microaneurysms containing foam cells); and quantification of mGA (panel B), mGV (panel C), glomerular matrix (panel D), foam cells (panel E), microaneurysms (panel F) and tubulo-interstitial fibrosis (panel G) in untreated and D-carnosine octylester-treated ApoE null mice fed a NFD or a HFD (mean ± SD; n_= 10 per group); scale bar = 50 µm. *P < 0.001 versus NFD-fed mice; †_P < 0.001 versus untreated mice. DCO = D-carnosine octylester.
Similar articles
- Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice.
Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Pugliese G. Menini S, et al. Diabetologia. 2015 Apr;58(4):845-53. doi: 10.1007/s00125-014-3467-6. Epub 2014 Dec 4. Diabetologia. 2015. PMID: 25471794 - FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice.
Iacobini C, Menini S, Blasetti Fantauzzi C, Pesce CM, Giaccari A, Salomone E, Lapolla A, Orioli M, Aldini G, Pugliese G. Iacobini C, et al. Br J Pharmacol. 2018 Jan;175(1):53-66. doi: 10.1111/bph.14070. Epub 2017 Dec 8. Br J Pharmacol. 2018. PMID: 29053168 Free PMC article. - Smooth Muscle Cell-Derived Interleukin-17C Plays an Atherogenic Role via the Recruitment of Proinflammatory Interleukin-17A+ T Cells to the Aorta.
Butcher MJ, Waseem TC, Galkina EV. Butcher MJ, et al. Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1496-506. doi: 10.1161/ATVBAHA.116.307892. Epub 2016 Jun 30. Arterioscler Thromb Vasc Biol. 2016. PMID: 27365405 Free PMC article. - L-carnosine and its Derivatives as New Therapeutic Agents for the Prevention and Treatment of Vascular Complications of Diabetes.
Menini S, Iacobini C, Fantauzzi CB, Pugliese G. Menini S, et al. Curr Med Chem. 2020;27(11):1744-1763. doi: 10.2174/0929867326666190711102718. Curr Med Chem. 2020. PMID: 31296153 Review. - Proatherogenic effects of 4-hydroxynonenal.
Nègre-Salvayre A, Garoby-Salom S, Swiader A, Rouahi M, Pucelle M, Salvayre R. Nègre-Salvayre A, et al. Free Radic Biol Med. 2017 Oct;111:127-139. doi: 10.1016/j.freeradbiomed.2016.12.038. Epub 2016 Dec 29. Free Radic Biol Med. 2017. PMID: 28040472 Review.
Cited by
- A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice.
Weigand T, Colbatzky F, Pfeffer T, Garbade SF, Klingbeil K, Colbatzky F, Becker M, Zemva J, Bulkescher R, Schürfeld R, Thiel C, Volk N, Reuss D, Hoffmann GF, Freichel M, Hecker M, Poth T, Fleming T, Poschet G, Schmitt CP, Peters V. Weigand T, et al. Int J Mol Sci. 2020 Jul 10;21(14):4887. doi: 10.3390/ijms21144887. Int J Mol Sci. 2020. PMID: 32664451 Free PMC article. - Chicken or Egg? Mitochondrial Phospholipids and Oxidative Stress in Disuse-Induced Skeletal Muscle Atrophy.
Miranda ER, Shahtout JL, Funai K. Miranda ER, et al. Antioxid Redox Signal. 2023 Feb;38(4-6):338-351. doi: 10.1089/ars.2022.0151. Antioxid Redox Signal. 2023. PMID: 36301935 Free PMC article. Review. - Food-Related Carbonyl Stress in Cardiometabolic and Cancer Risk Linked to Unhealthy Modern Diet.
Iacobini C, Vitale M, Haxhi J, Pesce C, Pugliese G, Menini S. Iacobini C, et al. Nutrients. 2022 Mar 3;14(5):1061. doi: 10.3390/nu14051061. Nutrients. 2022. PMID: 35268036 Free PMC article. Review. - The protective role of carnosine against type 2 diabetes-induced cognitive impairment.
Wang Q, Tripodi N, Valiukas Z, Bell SM, Majid A, de Courten B, Apostolopoulos V, Feehan J. Wang Q, et al. Food Sci Nutr. 2024 Mar 12;12(6):3819-3833. doi: 10.1002/fsn3.4077. eCollection 2024 Jun. Food Sci Nutr. 2024. PMID: 38873448 Free PMC article. Review. - Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future.
Iacobini C, Vitale M, Pesce C, Pugliese G, Menini S. Iacobini C, et al. Antioxidants (Basel). 2021 May 5;10(5):727. doi: 10.3390/antiox10050727. Antioxidants (Basel). 2021. PMID: 34063078 Free PMC article. Review.
References
- Aldini G, Maffei Facino R, Beretta G, Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors. 2005;24:77–87. - PubMed
- Aldini G, Dalle-Donne I, Colombo R, Maffei Facino R, Milzani A, Carini M. Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction. ChemMedChem. 2006;1:1045–1058. - PubMed
- Babizhayev MA, Deyev AI, Yermakova VN, Brikman IV, Bours J. Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R D. 2004;5:125–139. - PubMed
- Canevotti R, Negrisoli G, Previtali M. 2008. Dipeptide compounds containing D-histidine. WO2008001175(A2)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous