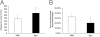VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression - PubMed (original) (raw)
VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression
Haibo Wang et al. Am J Pathol. 2012 Mar.
Abstract
Avascular, hypoxic retina has been postulated to be a source of angiogenic factors that cause aberrant angiogenesis and intravitreal neovascularization (IVNV) in retinopathy of prematurity. Vascular endothelial growth factor (VEGF) is an important factor involved. However, VEGF is also required for normal retinal vascular development, which raises concerns about inhibiting its activity to treat IVNV in retinopathy of prematurity. Therefore, understanding the effects that VEGF has on other factors in the development of avascular retina is important to prevent aberrant angiogenesis and IVNV. Here, we show that STAT3 was activated by increased retinal VEGF in the rat 50/10 oxygen-induced retinopathy model. Phospho-STAT3 colocalized with glutamine synthetase-labeled Müller cells. Inhibition of STAT3 reduced avascular retina and increased retinal erythropoietin (Epo) expression. Epo administered exogenously also reduced avascular retina in the model. In an in vitro study, hypoxia-induced VEGF inhibited Epo gene expression by STAT3 activation in rat Müller cells. The mechanism by which activated STAT3 regulated Epo was by inhibition of Epo promoter activity. Together, these findings show that increased retinal VEGF contributes to avascular retina by regulating retinal Epo expression through Janus kinase/STAT signaling. Our results suggest that rescuing Epo expression in the retina before the development of IVNV may promote normal developmental angiogenesis and, therefore, reduce the stimulus for later pathologic IVNV.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
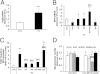
Figure 1
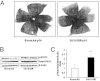
Pups raised in 50/10 OIR model have increased avascular retina and activation of retinal STAT3 at p14. A: Retinal vessels by flatmount with ADPase staining in pups raised in room air and 50/10 OIR model (n = 18). B: Western blot analysis of STAT3 phosphorylation in retina from pups raised in room air and 50/10 OIR. C: Quantification of Western gels shown in panel B. **P < 0.01 versus room air (ANOVA). Data shown in panels B and C are representative of 12 independent samples. Results are means ± SEMs.
Figure 2
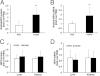
Decreased avascular retina with sustained retinal VEGF expression and caspase 3 activity in AG490-treated rat pups raised in the 50/10 OIR model. A: Western blot analysis of p-STAT3 and total STAT3 in retina. **P < 0.01 versus PBS (ANOVA; n = 8). B: Avascular retina analysis by measuring the ratio of avascular area to total retina area with the use of an image tool. *P < 0.01 versus PBS (ANOVA; n = 18). C and D: Western blot analyses of retinal VEGF protein (C) and active caspase 3 (D). Data shown in panels C and D are representative of 8 independent samples. Results are means ± SEMs.
Figure 3
Increased retinal Epo expression in AG490-treated rat pups raised in the 50/10 OIR model. A: Western blot analysis of Epo protein in retina. **P < 0.01 versus PBS (ANOVA; n = 7). B: Retinal Epo mRNA analysis by real-time PCR. **P < 0.01 versus PBS (ANOVA; n = 8). C: Western blot analysis of Epo protein in liver and kidney (n = 7). D: Real-time PCR of Epo mRNA in liver and kidney (n = 8). Results are means ± SEMs.
Figure 4
Decreased avascular retina by exogenous Epo given at early time points in the 50/10 OIR model. A: Platelet count of pups treated with Epo and PBS injection exposed to the 50/10 OIR model. *P < 0.05 versus PBS (ANOVA; n = 8). B: Analysis of avascular retina area. *P < 0.05 versus PBS (ANOVA; n = 8). Results are means ± SEMs.
Figure 5
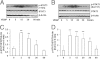
Decreased STAT3 phosphorylation and increased Epo expression in retinas of pups with VEGF-neutralizing antibody injection in the 50/10 OIR model. A and B: Retinal p-STAT3 measured by Western blot analysis with representative gels (A) and quantification of gels (B). Overall ANOVA, P < 0.001; post hoc Newman–Keuls multiple comparison testing: Vab versus Vab non-Inj, **P < 0.01, and Vab versus Vab IgG-Inj †††P < 0.001 (n = 6 to 8). C and D: Retinal Epo protein by Western blot analysis with representative gels (C) and quantification of gels (D). Overall ANOVA, P < 0.01; post hoc Newman–Keuls multiple comparison testing: Vab versus Vab non-Inj, **P < 0.01; Vab versus Vab IgG-Inj, ††P < 0.001 (n = 8). Results are means ± SEMs. IgG Inj, IgG injection; IgG Non, IgG noninjection; Vab Inj, VEGF antibody injection; Vab Non, VEGF antibody noninjection.
Figure 6
A: Increased p-STAT3 is localized in retinal Müller cells of pups raised in the 50/10 OIR model compared with pups raised in room air. Immunohistochemical labeling of cryosection that shows p-STAT3 colocalized with Müller cells (arrows) in room air and 50/10 OIR, using antibodies for p-STAT3 and Müller cell marker GS. No primary antibody control sections show mainly DAPI. Density of p-STAT3 colabeling with GS was quantified by confocal colocalization analysis and shows increased pSTAT3 in GS colabeled cells in the 50/10 OIR model compared with room air. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer. Representative of three separate experiments. B: Retinal Müller cells of pups raised in the 50/10 OIR model at p18 are hypoxic as shown by staining with antibody to GS and to Hypoxyprobe.
Figure 7
STAT3 is activated by VEGF in rat Müller cells (rMC-1). Western blot analysis of p-STAT3 at Tyr 705 (A and C) and Ser 727 (B and D), total STAT3 and loading control β-actin at different time points after VEGF (20 ng/mL) treatment (A and B). Representative quantification of gels (C and D). Overall ANOVA. P < 0.0001; post hoc Newman–Keuls multiple comparison testing: ***P < 0.001, **P < 0.01, and *P < 0.05 versus 0 minute of VEGF treatment. Results are means ± SEMs (n = 3). Data are representative of three independent experiments.
Figure 8
Activated STAT3 by hypoxia-induced VEGF suppresses Epo gene transcription by a STAT3 binding site within Epo promoter in rMC-1 cells. A and B: Real-time PCR of vegfa mRNA (A) and Epo mRNA (B) in rMC-1 cells exposed to 21% and 1% O2. Overall ANOVA, P < 0.0001; post hoc Newman–Keuls multiple comparison testing: *P < 0.05, ***P < 0.001, and ****P < 0.0001 versus 21% O2; †P < 0.05 and †††P < 0.001 versus 1% O2 Con. C: ChIP assay of STAT3 bound to a STAT3 binding site within a region of rat Epo promoter in rMC-1 cells exposed to 21% and 1% O2. Overall ANOVA, P < 0.0001; post hoc Newman-Keuls multiple comparison testing: ***P < 0.001 versus 21% O2, †P < 0.05, and †††P < 0.001 versus Con of 1% O2; ‡‡‡P < 0.001 versus AG, Vab or Vab+AG. D: Transient transfection of Cos-7 cells with Pluc-MCS reporter gene containing 300-bp promoter region from rat Epo gene with rat STAT3-expressing construct. Overall ANOVA, P < 0.001; post hoc Newman–Keuls multiple comparison testing: **P < 0.01 versus Con of Pluc-Epo_-promoter; †_P < 0.05 versus AG; ‡‡‡P < 0.001 versus VEGF+AG. AG, AG490; Con, control; Vab, VEGF antibody. Results are means ± SEMs (n = 3). Data are representative of two independent experiments.
Similar articles
- Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity.
McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. McCloskey M, et al. Invest Ophthalmol Vis Sci. 2013 Mar 21;54(3):2020-6. doi: 10.1167/iovs.13-11625. Invest Ophthalmol Vis Sci. 2013. PMID: 23449716 Free PMC article. - Anti-VEGF therapy in the management of retinopathy of prematurity: what we learn from representative animal models of oxygen-induced retinopathy.
Wang H. Wang H. Eye Brain. 2016 May 20;8:81-90. doi: 10.2147/EB.S94449. eCollection 2016. Eye Brain. 2016. PMID: 28539803 Free PMC article. Review. - Vascular Endothelial Growth Factor Signaling in Models of Oxygen-Induced Retinopathy: Insights Into Mechanisms of Pathology in Retinopathy of Prematurity.
Ramshekar A, Hartnett ME. Ramshekar A, et al. Front Pediatr. 2021 Dec 9;9:796143. doi: 10.3389/fped.2021.796143. eCollection 2021. Front Pediatr. 2021. PMID: 34956992 Free PMC article. Review.
Cited by
- Assessment of vascular regeneration in the CNS using the mouse retina.
Miloudi K, Dejda A, Binet F, Lapalme E, Cerani A, Sapieha P. Miloudi K, et al. J Vis Exp. 2014 Jun 23;(88):e51351. doi: 10.3791/51351. J Vis Exp. 2014. PMID: 24998265 Free PMC article. - Advanced glycation end (AGE) product modification of laminin downregulates Kir4.1 in retinal Müller cells.
Thompson K, Chen J, Luo Q, Xiao Y, Cummins TR, Bhatwadekar AD. Thompson K, et al. PLoS One. 2018 Feb 23;13(2):e0193280. doi: 10.1371/journal.pone.0193280. eCollection 2018. PLoS One. 2018. PMID: 29474462 Free PMC article. - Mechanisms and management of retinopathy of prematurity.
Hartnett ME, Penn JS. Hartnett ME, et al. N Engl J Med. 2012 Dec 27;367(26):2515-26. doi: 10.1056/NEJMra1208129. N Engl J Med. 2012. PMID: 23268666 Free PMC article. Review. No abstract available. - Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Müller cells maintains retinal function by triggering different signaling mechanisms.
Becker S, Wang H, Simmons AB, Suwanmanee T, Stoddard GJ, Kafri T, Hartnett ME. Becker S, et al. Sci Rep. 2018 Jan 31;8(1):2003. doi: 10.1038/s41598-018-20278-4. Sci Rep. 2018. PMID: 29386650 Free PMC article. - VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis.
Yang Z, Wang H, Jiang Y, Hartnett ME. Yang Z, et al. Am J Pathol. 2014 Apr;184(4):1230-1239. doi: 10.1016/j.ajpath.2013.12.023. Epub 2014 Mar 12. Am J Pathol. 2014. PMID: 24630601 Free PMC article.
References
- Chen J., Smith L.E. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. - PubMed
- Schaffer D.B., Palmer E.A., Plotsky D.F., Metz H.S., Flynn J.T., Tung B., Hardy R.J. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–237. - PubMed
- Aiello L.P. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. - PubMed
- Nicholson B., Schachat A. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY015130/EY/NEI NIH HHS/United States
- R01EY015130/EY/NEI NIH HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
- R01 EY017011/EY/NEI NIH HHS/United States
- R01EY017011/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous