The Malassezia genus in skin and systemic diseases - PubMed (original) (raw)
Review
The Malassezia genus in skin and systemic diseases
Georgios Gaitanis et al. Clin Microbiol Rev. 2012 Jan.
Abstract
In the last 15 years, the genus Malassezia has been a topic of intense basic research on taxonomy, physiology, biochemistry, ecology, immunology, and metabolomics. Currently, the genus encompasses 14 species. The 1996 revision of the genus resulted in seven accepted taxa: M. furfur, M. pachydermatis, M. sympodialis, M. globosa, M. obtusa, M. restricta, and M. slooffiae. In the last decade, seven new taxa isolated from healthy and lesional human and animal skin have been accepted: M. dermatis, M. japonica, M. yamatoensis, M. nana, M. caprae, M. equina, and M. cuniculi. However, forthcoming multidisciplinary research is expected to show the etiopathological relationships between these new species and skin diseases. Hitherto, basic and clinical research has established etiological links between Malassezia yeasts, pityriasis versicolor, and sepsis of neonates and immunocompromised individuals. Their role in aggravating seborrheic dermatitis, dandruff, folliculitis, and onychomycosis, though often supported by histopathological evidence and favorable antifungal therapeutic outcomes, remains under investigation. A close association between skin and Malassezia IgE binding allergens in atopic eczema has been shown, while laboratory data support a role in psoriasis exacerbations. Finally, metabolomic research resulted in the proposal of a hypothesis on the contribution of Malassezia-synthesized aryl hydrocarbon receptor (AhR) ligands to basal cell carcinoma through UV radiation-induced carcinogenesis.
Figures
Fig 1
Pityriasis versicolor in a 42-year-old female patient. The patient had relapsing disease for the past 6 years.
Fig 2
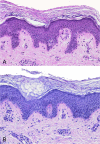
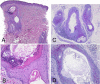
Histopathology of noninflammatory pityriasis versicolor. Shown is the infiltration of the hyperkeratotic stratum corneum by Malassezia cells and hyphae; there is a distinct absence of an inflammatory cell infiltrate. (A) Hematoxylin-eosin stain; (B) PAS stain. Original magnification, ×200.
Fig 3
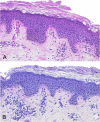
Histopathology of inflammatory pityriasis versicolor. Shown is the infiltration of the hyperkeratotic stratum corneum by Malassezia cells and hyphae; there is a moderately dense perivascular inflammatory cell infiltrate in the upper dermis. (A) Hematoxylin-eosin stain; (B) PAS stain. Original magnification, ×200.
Fig 4
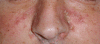
Seborrheic dermatitis in the nasolabial folds. The distribution of the lesions is typical; however, the seborrheic dermatitis can be characterized as severe, as the disease is extended into the parietal region and is associated with intense erythema and scaling.
Fig 5
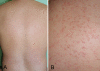
Malassezia folliculitis in a 34-year-old construction worker. The condition developed after working in a hot, humid environment for a few days. (A) Back of the patient. (B) Close-up view of the lesions.
Fig 6
Histopathology of Malassezia folliculitis. (A) Dilated hair follicle filled with keratinous material and basophilic debris. Shown is a perifollicular inflammatory cell infiltrate with hematoxylin-eosin staining. Original magnification, ×40. (B) Detail of panel A showing hardly recognizable yeast cells in this section in keratinous masses within the infundibular lumen adjacent to the site of wall destruction. Hematoxylin-eosin staining is shown. Original magnification, ×200. (C) Dense perifollicular chronic inflammatory cell infiltrate with amorphous mucinous material in the dilated follicle lumen and PAS stain-positive tiny budding yeasts. PAS staining was used. Original magnification, ×100. (D) Detail of a serial section of the same follicle demonstrating numerous yeast spores within the dilated follicle lumen. PAS staining was used. Original magnification, ×200.
Fig 7
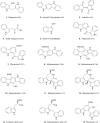
Chemical structures of the currently identified indoles produced by M. furfur when grown on
l
-tryptophan agar. The corresponding references of the first description of isolation from Malassezia extracts are in parentheses.
Fig 7
Chemical structures of the currently identified indoles produced by M. furfur when grown on
l
-tryptophan agar. The corresponding references of the first description of isolation from Malassezia extracts are in parentheses.
Similar articles
- [Malassezia infections].
Sei Y. Sei Y. Med Mycol J. 2012;53(1):7-11. doi: 10.3314/mmj.53.7. Med Mycol J. 2012. PMID: 22467125 Japanese. - Malassezia species in healthy skin and in dermatological conditions.
Prohic A, Jovovic Sadikovic T, Krupalija-Fazlic M, Kuskunovic-Vlahovljak S. Prohic A, et al. Int J Dermatol. 2016 May;55(5):494-504. doi: 10.1111/ijd.13116. Epub 2015 Dec 29. Int J Dermatol. 2016. PMID: 26710919 Review. - Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects.
Nakabayashi A, Sei Y, Guillot J. Nakabayashi A, et al. Med Mycol. 2000 Oct;38(5):337-41. doi: 10.1080/mmy.38.5.337.341. Med Mycol. 2000. PMID: 11092380 - [Examination of the causative agent of pityriasis versicolor].
Morishita N, Sei Y, Takiuchi I, Sugita T. Morishita N, et al. Nihon Ishinkin Gakkai Zasshi. 2005;46(3):169-70. doi: 10.3314/jjmm.46.169. Nihon Ishinkin Gakkai Zasshi. 2005. PMID: 16094290 Review. Japanese. - Isolation of Malassezia globosa and M. sympodialis from patients with pityriasis versicolor in Spain.
Aspiroz C, Ara M, Varea M, Rezusta A, Rubio C. Aspiroz C, et al. Mycopathologia. 2002;154(3):111-7. doi: 10.1023/a:1016020209891. Mycopathologia. 2002. PMID: 12171443
Cited by
- Mite allergy and atopic dermatitis: Is there a clear link? (Review).
Bumbacea RS, Corcea SL, Ali S, Dinica LC, Fanfaret IS, Boda D. Bumbacea RS, et al. Exp Ther Med. 2020 Oct;20(4):3554-3560. doi: 10.3892/etm.2020.9120. Epub 2020 Aug 13. Exp Ther Med. 2020. PMID: 32905207 Free PMC article. Review. - Malassezia arunalokei sp. nov., a Novel Yeast Species Isolated from Seborrheic Dermatitis Patients and Healthy Individuals from India.
Honnavar P, Prasad GS, Ghosh A, Dogra S, Handa S, Rudramurthy SM. Honnavar P, et al. J Clin Microbiol. 2016 Jul;54(7):1826-1834. doi: 10.1128/JCM.00683-16. Epub 2016 May 4. J Clin Microbiol. 2016. PMID: 27147721 Free PMC article. - Seborrheic dermatitis treatment with stellate ganglion block: a case report.
Kim GW, Mun KH, Song JY, Kim BG, Jung JK, Lee CS, Cha YD, Song JH. Kim GW, et al. Korean J Anesthesiol. 2016 Apr;69(2):171-4. doi: 10.4097/kjae.2016.69.2.171. Epub 2016 Mar 30. Korean J Anesthesiol. 2016. PMID: 27064785 Free PMC article. - Detection and identification of Malassezia species in domestic animals and aquatic birds by PCR-RFLP.
Zia M, Mirhendi H, Toghyani M. Zia M, et al. Iran J Vet Res. 2015 Winter;16(1):36-41. Iran J Vet Res. 2015. PMID: 27175148 Free PMC article. - Human Three-Dimensional Models for Studying Skin Pathogens.
Boero E, Mnich ME, Manetti AGO, Soldaini E, Grimaldi L, Bagnoli F. Boero E, et al. Curr Top Microbiol Immunol. 2021;430:3-27. doi: 10.1007/82_2020_219. Curr Top Microbiol Immunol. 2021. PMID: 32601967 Review.
References
- Adachi J, Mori Y, Matsui S, Matsuda T. 2004. Comparison of gene expression patterns between 2,3,7,8-tetrachlorodibenzo-p-dioxin and a natural arylhydrocarbon receptor ligand, indirubin. Toxicol. Sci. 80:161–169 - PubMed
- Agerberth B, et al. 2006. Malassezia sympodialis differently affects the expression of LL-37 in dendritic cells from atopic eczema patients and healthy individuals. Allergy 61:422–430 - PubMed
- Aizawa T, Kano R, Nakamura Y, Watanabe S, Hasegawa A. 2001. The genetic diversity of clinical isolates of Malassezia pachydermatis from dogs and cats. Med. Mycol. 39:329–334 - PubMed
- Aizawa T, Kano R, Nakamura Y, Watanabe S, Hasegawa A. 1999. Molecular heterogeneity in clinical isolates of Malassezia pachydermatis from dogs. Vet. Microbiol. 70:67–75 - PubMed
- Akaza N, et al. 2009. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med. Mycol. 47:618–624 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical