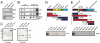Interplay between mismatch repair and chromatin assembly - PubMed (original) (raw)
Interplay between mismatch repair and chromatin assembly
Barbara Schöpf et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Single strand nicks and gaps in DNA have been reported to increase the efficiency of nucleosome loading mediated by chromatin assembly factor 1 (CAF-1). However, on mismatch-containing substrates, these strand discontinuities are utilized by the mismatch repair (MMR) system as loading sites for exonuclease 1, at which degradation of the error-containing strand commences. Because packaging of DNA into chromatin might inhibit MMR, we were interested to learn whether chromatin assembly is differentially regulated on heteroduplex and homoduplex substrates. We now show that the presence of a mismatch in a nicked plasmid substrate delays nucleosome loading in human cell extracts. Our data also suggest that, once the mismatch is removed, repair of the single-stranded gap is accompanied by efficient nucleosome loading. We postulated that the balance between MMR and chromatin assembly might be governed by proliferating cell nuclear antigen (PCNA), the processivity factor of replicative DNA polymerases, which is loaded at DNA termini and which interacts with the MSH6 subunit of the mismatch recognition factor MutSα, as well as with CAF-1. We now show that this regulation might be more complex; MutSα and CAF-1 interact not only with PCNA, but also with each other. In vivo this interaction increases during S-phase and may be controlled by the phosphorylation status of the p150 subunit of CAF-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
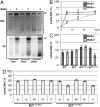
Ongoing MMR delays chromatin assembly. (A) Kinetics of supercoiling of nicked mismatch-containing (G/Tnicked) DNA substrates incubated with nuclear extracts of human MutSα-deficient LoVo cells, supplemented where indicated with recombinant MutSα. The figure shows a UV shadowing of an agarose gel poststained with EtBr (Upper) and its autoradiograph (32P, Lower). Migration positions of the open/nicked circular (Ir/II) and supercoiled DNA (I) isoforms are indicated. (B) Quantitation of three independent G/Tnicked supercoiling experiments (A shows a representative example), as a ratio of the sum of all covalently closed topoisomers (cc) versus total DNA. (C) Supercoiling of homoduplex (A/T, A/Tnicked) and heteroduplex (G/T, G/Tnicked) substrates incubated with nuclear extracts of human MutSα-deficient LoVo cells, supplemented where indicated with recombinant MutSα. Data from three independent experiments are shown. The error bars represent the standard deviation from the mean. (D) Quantitation of supercoiling assays of A/Tnicked and G/Tnicked substrates after incubation with nuclear extracts of human MutSα-deficient LoVo cells, supplemented where indicated with recombinant MutSα—either wild type, or its variants KR (ATPase mutant), FA (DNA binding mutant), or C1 (PCNA interaction mutant). Data from three independent experiments were analyzed. The error bars represent the standard deviation from the mean.
Fig. 2.
Effect of CAF-1 on the efficiency of chromatin assembly and MMR. (A) Representative supercoiling/repair assay of A/Tnicked and G/Tnicked substrates in nuclear extracts of LoVo cells supplemented with MutSα and/or with recombinant CAF-1 as indicated. (B) MMR efficiency in the extracts was estimated by recovering the G/Tnicked substrates following incubation with the extracts and digestion with _Acl_I. As shown, addition of CAF-1 to the assay did not alter MMR efficiency. (C) After 10 min preincubation (+) of the A/Tnicked and G/Tnicked substrates with LoVo extracts supplemented (+) or not (−) with MutSα. In the control experiments (−), the extract was incubated under identical conditions, and the substrates were added after 10 min together with the MutSα. The extent of supercoiling was examined 15 min later. Data from three independent experiments were analyzed. The error bars represent the standard deviation from the mean. (D) MMR assay of the G/Tnicked substrate preincubated with (+) or without (−) MutSα as indicated.
Fig. 3.
MutSα interacts directly with the p150 subunit of CAF-1. (A) Anti-MSH6 coimmunoprecipitations were analyzed by Western blotting for CAF-1. Ten percent HeLa nuclear extracts served as the input control. (B) Extracts of 293 cells stably expressing FLAG-CAF-1 p150 were incubated with anti-FLAG beads. Elution was done using FLAG peptides. The control was 293 cells expressing FLAG only (empty). The input control was 0.5% of eluted material. Input fraction (I), Unbound material (U), Eluate (E). Asterisk marks leftover signal of previous p150 blot. (C) Far Western blot showing a direct interaction between MutSα and CAF-1. The CAF-1 trimer was separated by SDS-PAGE, transferred onto a membrane, incubated with MutSα and hybridized with an anti-MSH6 antibody. BSA was used as negative control. (D) Schematic representation of human MSH6. The PWWP (red), DNA-binding (orange), and ATPase (light blue) domains are indicated. The clamp region (dark green) is located within the lever domain (light green) that follows the connector domain (yellow). The GST-MSH6 fusion fragments that interacted with purified p150 in a GST pull-down experiment are shown in red, fragments that did not interact are in blue. (E) Schematic representation of the p150 subunit of human CAF-1. MIR (MOD1-interacting region), PEST (yellow), as well as KER (red), and ED (green) histone interacting domains are shown. The PCNA-binding motif (orange) as well as the p60-interacting region (light blue) are also indicated. The C-terminal half of p150 is needed for replication-coupled assembly. The GST-p150 fragments that interacted with purified MutSα in a GST pull-down experiment are indicated in red, those that did not interact are shown in blue.
Fig. 4.
Interactions between MutSα, CAF-1, and PCNA are differentially affected by treatment with DNA damaging agents. (A) Cell extracts from different cell cycle stages (
Fig. S4_A_
) were immunoprecipitated with an anti-MSH6 antibody and analyzed by Western blotting. (B) U2OS cells were pretreated with _O_6-benzylguanine to inhibit methylguanine methyl transferase before treatment with 10 μM MNNG. Anti-MSH6 immunoprecipitates were analyzed for MSH6, CAF-1 p150, or PCNA. (C) Lambda phosphatase treated or untreated purified, recombinant MutSα and CAF-1 p150 were used in immunoprecipitations with an anti-MSH6 antibody. The prefix p indicates polypeptides endogenously phosphorylated in Sf9 cells.
Similar articles
- A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage.
Moggs JG, Grandi P, Quivy JP, Jónsson ZO, Hübscher U, Becker PB, Almouzni G. Moggs JG, et al. Mol Cell Biol. 2000 Feb;20(4):1206-18. doi: 10.1128/MCB.20.4.1206-1218.2000. Mol Cell Biol. 2000. PMID: 10648606 Free PMC article. - DNA Mismatch Repair Interacts with CAF-1- and ASF1A-H3-H4-dependent Histone (H3-H4)2 Tetramer Deposition.
Rodriges Blanko E, Kadyrova LY, Kadyrov FA. Rodriges Blanko E, et al. J Biol Chem. 2016 Apr 22;291(17):9203-17. doi: 10.1074/jbc.M115.713271. Epub 2016 Mar 4. J Biol Chem. 2016. PMID: 26945061 Free PMC article. - CAF-I-dependent control of degradation of the discontinuous strands during mismatch repair.
Kadyrova LY, Blanko ER, Kadyrov FA. Kadyrova LY, et al. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2753-8. doi: 10.1073/pnas.1015914108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282622 Free PMC article. - Chromatin assembly during S phase: contributions from histone deposition, DNA replication and the cell division cycle.
Krude T, Keller C. Krude T, et al. Cell Mol Life Sci. 2001 May;58(5-6):665-72. doi: 10.1007/pl00000890. Cell Mol Life Sci. 2001. PMID: 11437228 Free PMC article. Review. - The ins and outs of nucleosome assembly.
Mello JA, Almouzni G. Mello JA, et al. Curr Opin Genet Dev. 2001 Apr;11(2):136-41. doi: 10.1016/s0959-437x(00)00170-2. Curr Opin Genet Dev. 2001. PMID: 11250135 Review.
Cited by
- Understanding how mismatch repair proteins participate in the repair/anti-recombination decision.
Chakraborty U, Alani E. Chakraborty U, et al. FEMS Yeast Res. 2016 Sep;16(6):fow071. doi: 10.1093/femsyr/fow071. Epub 2016 Aug 28. FEMS Yeast Res. 2016. PMID: 27573382 Free PMC article. - Regulation of oxidized base damage repair by chromatin assembly factor 1 subunit A.
Yang C, Sengupta S, Hegde PM, Mitra J, Jiang S, Holey B, Sarker AH, Tsai MS, Hegde ML, Mitra S. Yang C, et al. Nucleic Acids Res. 2017 Jan 25;45(2):739-748. doi: 10.1093/nar/gkw1024. Epub 2016 Oct 27. Nucleic Acids Res. 2017. PMID: 27794043 Free PMC article. - Chromatin Modifiers Alter Recombination Between Divergent DNA Sequences.
Chakraborty U, Mackenroth B, Shalloway D, Alani E. Chakraborty U, et al. Genetics. 2019 Aug;212(4):1147-1162. doi: 10.1534/genetics.119.302395. Epub 2019 Jun 20. Genetics. 2019. PMID: 31221666 Free PMC article. - The unstructured linker arms of MutL enable GATC site incision beyond roadblocks during initiation of DNA mismatch repair.
Mardenborough YSN, Nitsenko K, Laffeber C, Duboc C, Sahin E, Quessada-Vial A, Winterwerp HHK, Sixma TK, Kanaar R, Friedhoff P, Strick TR, Lebbink JHG. Mardenborough YSN, et al. Nucleic Acids Res. 2019 Dec 16;47(22):11667-11680. doi: 10.1093/nar/gkz834. Nucleic Acids Res. 2019. PMID: 31598722 Free PMC article. - Decoding the histone code: Role of H3K36me3 in mismatch repair and implications for cancer susceptibility and therapy.
Li GM. Li GM. Cancer Res. 2013 Nov 1;73(21):6379-83. doi: 10.1158/0008-5472.CAN-13-1870. Epub 2013 Oct 21. Cancer Res. 2013. PMID: 24145353 Free PMC article. Review.
References
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. - PubMed
- Jiricny J, et al. Mismatch repair defects in cancer. Curr Opin Genet Dev. 2000;10:157–161. - PubMed
- Moldovan GL, et al. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. - PubMed
- Smith S, et al. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous