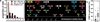A novel retinoblastoma therapy from genomic and epigenetic analyses - PubMed (original) (raw)
. 2012 Jan 11;481(7381):329-34.
doi: 10.1038/nature10733.
Claudia A Benavente, Justina McEvoy, Jacqueline Flores-Otero, Li Ding, Xiang Chen, Anatoly Ulyanov, Gang Wu, Matthew Wilson, Jianmin Wang, Rachel Brennan, Michael Rusch, Amity L Manning, Jing Ma, John Easton, Sheila Shurtleff, Charles Mullighan, Stanley Pounds, Suraj Mukatira, Pankaj Gupta, Geoff Neale, David Zhao, Charles Lu, Robert S Fulton, Lucinda L Fulton, Xin Hong, David J Dooling, Kerri Ochoa, Clayton Naeve, Nicholas J Dyson, Elaine R Mardis, Armita Bahrami, David Ellison, Richard K Wilson, James R Downing, Michael A Dyer
Affiliations
- PMID: 22237022
- PMCID: PMC3289956
- DOI: 10.1038/nature10733
A novel retinoblastoma therapy from genomic and epigenetic analyses
Jinghui Zhang et al. Nature. 2012.
Abstract
Retinoblastoma is an aggressive childhood cancer of the developing retina that is initiated by the biallelic loss of RB1. Tumours progress very quickly following RB1 inactivation but the underlying mechanism is not known. Here we show that the retinoblastoma genome is stable, but that multiple cancer pathways can be epigenetically deregulated. To identify the mutations that cooperate with RB1 loss, we performed whole-genome sequencing of retinoblastomas. The overall mutational rate was very low; RB1 was the only known cancer gene mutated. We then evaluated the role of RB1 in genome stability and considered non-genetic mechanisms of cancer pathway deregulation. For example, the proto-oncogene SYK is upregulated in retinoblastoma and is required for tumour cell survival. Targeting SYK with a small-molecule inhibitor induced retinoblastoma tumour cell death in vitro and in vivo. Thus, retinoblastomas may develop quickly as a result of the epigenetic deregulation of key cancer pathways as a direct or indirect result of RB1 loss.
Figures
Figure 1. Characterization of retinoblastomas samples
a–c, Representative retinoblastoma tumor section(SJRB001) stained with hematoxylin and eosin (H&E) showing choroidal and optic nerve invasion (arrow). d–f, H&E-stained section of the SJRB001X orthotopic xenograft with choroidal (e) and optic nerve (f) invasion (arrows). Abbreviations: AC, anterior chamber; ON, optic nerve; Sc, sclera. Scale bars: 25 µm.
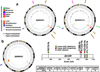
Figure 2. Genomic profiles of SJRB001-2 and SJRB001X
a,b, CIRCOS plots of genetic alterations in 2 retinoblastomas and the matched orthotopic xenograft. Loss of heterozygosity (orange), amplifications (red), and deletions (blue) are shown. Interchromosomal translocations (green lines) and intrachromosomal translocations (purple lines) are indicated. Sequence mutations in Refseq genes included silent single nucleotide variants (SNVs, green), missense SNVs (brown), nonsense SNVs (dark blue), splice-site mutations (pink), and insertion/deletion mutations (indels, red). c) BCOR mutations identified in the recurrency cohort.
Figure 3. Analysis of aneuploidy and CIN in retinoblastoma
a, Chromosomal missegregation of SJRB001X cells after at least 21 rounds of cell division is plotted in red. b, Representative SKY image of SJRB001X after the third passage in mice. c, Alterations in the 46 Rb cases (Rb) compared to 153 high-grade serous ovarian cancer (Ov) from TGCA. The median chromosomal lesions for retinoblastoma (Rb) was 1.5% and 27.7% for ovarian cancer (Ov).
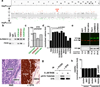
Figure 4. SYK Is Expressed in Retinoblastoma and Is Required for Survival
a, Whole-genome view of the gene ranks based on integrating ChIP-on-chip, methylation, and gene expression results. Y-axis is –log(p), where p is a p-value of Q-statistic corrected for multiple testing. Significantly (FDR ≤10%) downregulated (green) or upregulated (red) genes are shown. b, c, ChIP validation of histone markers of the SYK promoter including quantification by quantitative PCR (qPCR) with TaqMan probes. Each bar is the mean and standard deviation of triplicate samples. d, SYK gene expression measured by qPCR in fetal week 20 retina (fetal), primary retinoblastoma (tumor), orthotopic xenografts (SJRB001X and SJRB002X) and cell lines. Each bar is the mean and standard deviation of duplicate samples normalized to GPI1 expression. e, Immunoblot of SYK (green) and actin (red) in orthotopic xenografts, human fetal retina, and representative cell lines; black and white representation of the SYK immunoblot is in the lower panel. f, H&E (purple) and anti-SYK (brown) immunohistochemistry of retinoblastoma tissue. g, Immunoprecipitation analysis of SYK and pSYK Y525/526 from Weri1 retinoblastoma cells. h, Viability was measured in triplicate cultures 72 hours after infection of retinoblastoma cells with a lentivirus vector expressing either a control lentivirus or an shRNA against SYK. Scale bars: 10 µm.
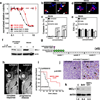
Figure 5. Retinoblastoma Cells are Sensitive to SYK Inhibitors
a, Dose response for SYK inhibitors R406 (red) and BAY 61-3606 (black) in RB355 retinoblastoma cells and a negative control (Jurkat). Each data point is the mean and standard deviation of triplicate samples. b–e Immunofluorescence of activated caspase 3 or EdU(red) before and after treatment of RB355 cells with R406 or BAY 61–3606. A total of 250 cells were scored in duplicate for each sample and each treatment condition to derive the mean and standard deviation. Nuclei were stained with DAPI (blue). f, Treatment of stimulated Jurkat or RB355 cells with 5 µM BAY 61–3606 for 24 hours reduced MCL1 expression. g, Schematic of the treatment schedule for mice with SJRB001X tumors. h, Representative MR images of a mouse whose tumor responded after 4 courses of treatment with BAY 61–3606 (left) and another whose disease progressed during treatment (right). i, Survival curves show that BAY 61–3606+TPT treatment improved outcome. j, Immunostaining for activated caspase 3 (arrows) in untreated or BAY 61–3606–treated eyes. k, untreated or BAY 61-3606–treated eyes. k, Immunoblot showing reduced MCL1 protein after BAY 61–3606 or BAY 61–3606+TPT treatment. Scale bars b, d: 5 µm; j: 10 µm.
Comment in
- Genomics: The path to retinoblastoma.
Sage J, Cleary ML. Sage J, et al. Nature. 2012 Jan 18;481(7381):269-70. doi: 10.1038/481269a. Nature. 2012. PMID: 22258599 Free PMC article. - Retinoblastoma: Epigenetic outcome.
McCarthy N. McCarthy N. Nat Rev Cancer. 2012 Jan 24;12(2):80. doi: 10.1038/nrc3222. Nat Rev Cancer. 2012. PMID: 22270947 No abstract available. - Retinoblastoma tumorigenesis: genetic and epigenetic changes walk hand in hand.
Temming P, Corson TW, Lohmann DR. Temming P, et al. Future Oncol. 2012 May;8(5):525-8. doi: 10.2217/fon.12.41. Future Oncol. 2012. PMID: 22646767
Similar articles
- RB1 gene inactivation by chromothripsis in human retinoblastoma.
McEvoy J, Nagahawatte P, Finkelstein D, Richards-Yutz J, Valentine M, Ma J, Mullighan C, Song G, Chen X, Wilson M, Brennan R, Pounds S, Becksfort J, Huether R, Lu C, Fulton RS, Fulton LL, Hong X, Dooling DJ, Ochoa K, Mardis ER, Wilson RK, Easton J, Zhang J, Downing JR, Ganguly A, Dyer MA. McEvoy J, et al. Oncotarget. 2014 Jan 30;5(2):438-50. doi: 10.18632/oncotarget.1686. Oncotarget. 2014. PMID: 24509483 Free PMC article. - Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies.
Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, Pang R, Massey C, Godbout R, Jiang Z, Zacksenhaus E, Paton K, Moll AC, Houdayer C, Raizis A, Halliday W, Lam WL, Boutros PC, Lohmann D, Dorsman JC, Gallie BL. Rushlow DE, et al. Lancet Oncol. 2013 Apr;14(4):327-34. doi: 10.1016/S1470-2045(13)70045-7. Epub 2013 Mar 13. Lancet Oncol. 2013. PMID: 23498719 - Cross-species genomic and epigenomic landscape of retinoblastoma.
Benavente CA, McEvoy JD, Finkelstein D, Wei L, Kang G, Wang YD, Neale G, Ragsdale S, Valentine V, Bahrami A, Temirov J, Pounds S, Zhang J, Dyer MA. Benavente CA, et al. Oncotarget. 2013 Jun;4(6):844-59. doi: 10.18632/oncotarget.1051. Oncotarget. 2013. PMID: 23765217 Free PMC article. - Genetics and epigenetics of human retinoblastoma.
Benavente CA, Dyer MA. Benavente CA, et al. Annu Rev Pathol. 2015;10:547-62. doi: 10.1146/annurev-pathol-012414-040259. Annu Rev Pathol. 2015. PMID: 25621664 Review. - Progress in Small Molecule Therapeutics for the Treatment of Retinoblastoma.
Pritchard EM, Dyer MA, Guy RK. Pritchard EM, et al. Mini Rev Med Chem. 2016;16(6):430-54. doi: 10.2174/1389557515666150722100610. Mini Rev Med Chem. 2016. PMID: 26202204 Free PMC article. Review.
Cited by
- Long Noncoding RNA ZFPM2-AS1 Knockdown Restrains the Development of Retinoblastoma by Modulating the MicroRNA-515/HOXA1/Wnt/β-Catenin Axis.
Lyv X, Wu F, Zhang H, Lu J, Wang L, Ma Y. Lyv X, et al. Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):41. doi: 10.1167/iovs.61.6.41. Invest Ophthalmol Vis Sci. 2020. PMID: 32561925 Free PMC article. - Whole-Genome Sequencing of Retinoblastoma Reveals the Diversity of Rearrangements Disrupting RB1 and Uncovers a Treatment-Related Mutational Signature.
Davies HR, Broad KD, Onadim Z, Price EA, Zou X, Sheriff I, Karaa EK, Scheimberg I, Reddy MA, Sagoo MS, Ohnuma SI, Nik-Zainal S. Davies HR, et al. Cancers (Basel). 2021 Feb 11;13(4):754. doi: 10.3390/cancers13040754. Cancers (Basel). 2021. PMID: 33670346 Free PMC article. - Drosophila as a Potential Model for Ocular Tumors.
Bennett D, Lyulcheva E, Cobbe N. Bennett D, et al. Ocul Oncol Pathol. 2015 Apr;1(3):190-9. doi: 10.1159/000370155. Epub 2015 Apr 9. Ocul Oncol Pathol. 2015. PMID: 27172095 Free PMC article. Review. - Integrative analysis reveals relationships of genetic and epigenetic alterations in osteosarcoma.
Kresse SH, Rydbeck H, Skårn M, Namløs HM, Barragan-Polania AH, Cleton-Jansen AM, Serra M, Liestøl K, Hogendoorn PC, Hovig E, Myklebost O, Meza-Zepeda LA. Kresse SH, et al. PLoS One. 2012;7(11):e48262. doi: 10.1371/journal.pone.0048262. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144859 Free PMC article. - Evolution of the cancer genome.
Yates LR, Campbell PJ. Yates LR, et al. Nat Rev Genet. 2012 Nov;13(11):795-806. doi: 10.1038/nrg3317. Epub 2012 Oct 9. Nat Rev Genet. 2012. PMID: 23044827 Free PMC article. Review.
References
- Friend SH, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323:643–646. - PubMed
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
- Hernando E, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM81607/GM/NIGMS NIH HHS/United States
- R01 EY018599/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 EY018599-03/EY/NEI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- EY018599/EY/NEI NIH HHS/United States
- EY014867/EY/NEI NIH HHS/United States
- R01 GM081607/GM/NIGMS NIH HHS/United States
- CA64402/CA/NCI NIH HHS/United States
- R01 CA155202/CA/NCI NIH HHS/United States
- CA21765/CA/NCI NIH HHS/United States
- R01 EY014867/EY/NEI NIH HHS/United States
- R01 CA064402/CA/NCI NIH HHS/United States
- R01 EY014867-02/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous