Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients - PubMed (original) (raw)
Review
Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients
Michael Levin. Bioessays. 2012 Mar.
Abstract
Significant progress in the molecular investigation of endogenous bioelectric signals during pattern formation in growing tissues has been enabled by recently developed techniques. Ion flows and voltage gradients produced by ion channels and pumps are key regulators of cell proliferation, migration, and differentiation. Now, instructive roles for bioelectrical gradients in embryogenesis, regeneration, and neoplasm are being revealed through the use of fluorescent voltage reporters and functional experiments using well-characterized channel mutants. Transmembrane voltage gradients (V(mem) ) determine anatomical polarity and function as master regulators during appendage regeneration and embryonic left-right patterning. A state-of-the-art recent study reveals that they can also serve as prepatterns for gene expression domains during craniofacial patterning. Continued development of novel tools and better ways to think about physical controls of cell-cell interactions will lead to mastery of the morphogenetic information stored in physiological networks. This will enable fundamental advances in basic understanding of growth and form, as well as transformative biomedical applications in regenerative medicine.
Copyright © 2012 WILEY Periodicals, Inc.
Figures
Figure 1
Control of cell state by transmembrane potential. A sample of physiological measurements of various cell types (modified from [108]) reveals that quiescent, terminally differentiated cells tend to be strongly polarized, while more plastic cell types (stem cells, embryonic cells, and cancer cells) tend to be relatively depolarized. Interestingly, the liver's Vmem (abnormally low for an adult differentiated tissue) groups it with the morphogenetically labile cells, consistent with its remarkable regenerative potential. The relationship between Vmem and plasticity is a functional one; for example, mature neurons can be induced to re-enter the cell cycle by forced depolarization [109].
Figure 2
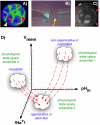
Voltage gradients in vivo. A: Fluorescent voltage reporter dyes allow characterization of physiological gradients in vivo, such as this image of a 16-cell frog embryo that simultaneously reveals cells' potential levels (blue = hyperpolarized, red = depolarized) in vivo, as well as domains of distinct Vmem around a
single
blastomere's surface (compare the side indicated by the yellow arrowhead with the one indicated by the red arrowhead). B: Gradients of transmembrane potential demarcate important tissue domains, such as the depolarized region shortly after tail amputation in Xenopus laevis tadpoles (blue arrowhead), which will give rise to the regeneration bud, and can reveal non-regenerative conditions when the appropriate physiological state had not been achieved, or was experimentally blocked (yellow arrowhead). C: Isopotential cell fields can also demarcate subtle prepatterns existing in tissues, such as the hyperpolarized domains (red arrowheads) that presage the expression of regulatory genes such as Frizzled during frog embryo craniofacial development [13]. It is necessary to gain a quantitative understanding of the bioelectric code – to map out the linkage between physiological state with cell behavior outcomes, as a prelude to a full understanding of how 3-dimensional patterning information is stored in physiological properties of tissue. D: One hypothesis is that cell types (e.g. proliferative, or neoplastic, or undifferentiated) cluster in a multi-dimensional state space in which each axis defines the value of a physiological parameter. Additional axes (not shown) could include levels of other ions (chloride, potassium), nuclear membrane potential, cell surface charge (zeta potentials), etc. Once appropriate data are gathered, cells could be moved from their current state to a desired state by pharmacological and molecular-genetic changes shifting them along each axis, toward a different ensemble within the state space as needed for a given biomedical application.
Figure 3
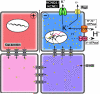
A framework for modeling bioelectrical signaling. A comprehensive model synthesizing bioelectrical and genetic pathway elements must integrate physiological descriptions of the ion channels and pumps expressed in cells into a quantitative picture of the voltage gradients, their effects on movement of small signaling molecules through gap junctions and membrane transporters, and ultimately effects on second messenger pathways and transcriptional responses. Here is shown a representative system (motivated by current models of early left-right patterning of frog embryos; the schematic was modified after Fig. 7B of [110] drawn by Junji Morokuma). Differential expression and function of well-characterized channels and pumps (e.g. V-ATPase, KCNQ1, and Katp) form circuits that establish distinct Vmem levels in different cells; this process can be mathematically modeled (as has been done for kidney, lens, and inner ear tissues) based on the known physiological properties of the translocators involved. The resulting voltage gradients gate gap junction connectivity states between adjacent cells, as well as exert electromotive force that regulates the movement of small signaling molecules (e.g. 5HT serotonin). This movement can be simulated using particle-tracking or differential equations. The resulting morphogen gradients can activate transcriptional changes, setting up prepatterns of gene expression that mirror the earlier voltage gradients, regionalizing tissues and closing the link between bioelectric events and specification of anatomy.
Figure 4
Physiological profiling. A: Cells exhibiting very different expression profiles for ion transporter genes can indeed be in similar physiological states (B), based on overlapping functions for the transporters. Lack of 1:1 mapping between expression of channels/pumps and physiological state (due to compensation by other family members and post-translational gating) means that important information about cell behavior is not captured by molecular-genetic analysis without physiological profiling. Nevertheless, transcriptional data can suggest hypotheses for functional validation: the GEO database [111] can be mined for ion channel/pump profiles, such as the increased expression of the Clic6 chloride channel during HSC maturation (C), and the changes in the expression of sodium channels during progression towards melanoma in skin cells (D).
Similar articles
- Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration.
Levin M. Levin M. J Physiol. 2014 Jun 1;592(11):2295-305. doi: 10.1113/jphysiol.2014.271940. J Physiol. 2014. PMID: 24882814 Free PMC article. Review. - Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering.
Levin M, Stevenson CG. Levin M, et al. Annu Rev Biomed Eng. 2012;14:295-323. doi: 10.1146/annurev-bioeng-071811-150114. Annu Rev Biomed Eng. 2012. PMID: 22809139 Free PMC article. Review. - Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo.
Levin M. Levin M. Mol Biol Cell. 2014 Dec 1;25(24):3835-50. doi: 10.1091/mbc.E13-12-0708. Mol Biol Cell. 2014. PMID: 25425556 Free PMC article. - Bioelectric mechanisms in regeneration: Unique aspects and future perspectives.
Levin M. Levin M. Semin Cell Dev Biol. 2009 Jul;20(5):543-56. doi: 10.1016/j.semcdb.2009.04.013. Epub 2009 May 3. Semin Cell Dev Biol. 2009. PMID: 19406249 Free PMC article. Review. - Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form.
McLaughlin KA, Levin M. McLaughlin KA, et al. Dev Biol. 2018 Jan 15;433(2):177-189. doi: 10.1016/j.ydbio.2017.08.032. Epub 2017 Dec 25. Dev Biol. 2018. PMID: 29291972 Free PMC article. Review.
Cited by
- Bioelectrical regulation of cell cycle and the planarian model system.
Barghouth PG, Thiruvalluvan M, Oviedo NJ. Barghouth PG, et al. Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2629-37. doi: 10.1016/j.bbamem.2015.02.024. Epub 2015 Mar 6. Biochim Biophys Acta. 2015. PMID: 25749155 Free PMC article. Review. - Kv3.1 channels stimulate adult neural precursor cell proliferation and neuronal differentiation.
Yasuda T, Cuny H, Adams DJ. Yasuda T, et al. J Physiol. 2013 May 15;591(10):2579-91. doi: 10.1113/jphysiol.2012.249151. Epub 2013 Mar 11. J Physiol. 2013. PMID: 23478135 Free PMC article. - Bioelectric Potential in Next-Generation Organoids: Electrical Stimulation to Enhance 3D Structures of the Central Nervous System.
O'Hara-Wright M, Mobini S, Gonzalez-Cordero A. O'Hara-Wright M, et al. Front Cell Dev Biol. 2022 May 17;10:901652. doi: 10.3389/fcell.2022.901652. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656553 Free PMC article. Review. - From the Microbiome to the Electrome: Implications for the Microbiota-Gut-Brain Axis.
Bourqqia-Ramzi M, Mansilla-Guardiola J, Muñoz-Rodriguez D, Quarta E, Lombardo-Hernandez J, Murciano-Cespedosa A, Conejero-Meca FJ, Mateos González Á, Geuna S, Garcia-Esteban MT, Herrera-Rincon C. Bourqqia-Ramzi M, et al. Int J Mol Sci. 2024 Jun 5;25(11):6233. doi: 10.3390/ijms25116233. Int J Mol Sci. 2024. PMID: 38892419 Free PMC article. - The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation.
Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, Harrison BS. Ross CL, et al. Stem Cell Res. 2015 Jul;15(1):96-108. doi: 10.1016/j.scr.2015.04.009. Epub 2015 May 12. Stem Cell Res. 2015. PMID: 26042793 Free PMC article. Review.
References
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. - PubMed
- Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–82. - PubMed
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–76. - PubMed
- Robinson K, Messerli M. Electric embryos: the embryonic epithelium as a generator of developmental information. In: McCaig C, editor. Nerve Growth and Guidance. Portland Press; Portland: 1996. pp. 131–41.
- Jenkins LS, Duerstock BS, Borgens RB. Reduction of the current of injury leaving the amputation inhibits limb regeneration in the red spotted newt. Dev Biol. 1996;178:251–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY018168/EY/NEI NIH HHS/United States
- R01 GM078484/GM/NIGMS NIH HHS/United States
- EY018168/EY/NEI NIH HHS/United States
- AR061988/AR/NIAMS NIH HHS/United States
- GM078484/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources