Fetal testosterone influences sexually dimorphic gray matter in the human brain - PubMed (original) (raw)
Fetal testosterone influences sexually dimorphic gray matter in the human brain
Michael V Lombardo et al. J Neurosci. 2012.
Abstract
In nonhuman species, testosterone is known to have permanent organizing effects early in life that predict later expression of sex differences in brain and behavior. However, in humans, it is still unknown whether such mechanisms have organizing effects on neural sexual dimorphism. In human males, we show that variation in fetal testosterone (FT) predicts later local gray matter volume of specific brain regions in a direction that is congruent with sexual dimorphism observed in a large independent sample of age-matched males and females from the NIH Pediatric MRI Data Repository. Right temporoparietal junction/posterior superior temporal sulcus (RTPJ/pSTS), planum temporale/parietal operculum (PT/PO), and posterior lateral orbitofrontal cortex (plOFC) had local gray matter volume that was both sexually dimorphic and predicted in a congruent direction by FT. That is, gray matter volume in RTPJ/pSTS was greater for males compared to females and was positively predicted by FT. Conversely, gray matter volume in PT/PO and plOFC was greater in females compared to males and was negatively predicted by FT. Subregions of both amygdala and hypothalamus were also sexually dimorphic in the direction of Male > Female, but were not predicted by FT. However, FT positively predicted gray matter volume of a non-sexually dimorphic subregion of the amygdala. These results bridge a long-standing gap between human and nonhuman species by showing that FT acts as an organizing mechanism for the development of regional sexual dimorphism in the human brain.
Figures
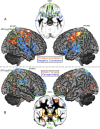
Figure 1.
FT correlations with local GM volume. A, Areas where FT predicts local gray matter volume. Red/orange voxels denote positive correlations; blue voxels denote negative correlations. B, Areas of sexual dimorphism in local GM volume. Red/orange voxels denote a Male > Female pattern; blue voxels denote a Female > Male pattern.
Figure 2.
Overlap of FT–GM correlations and sexual dimorphism. A, Conjunction analysis overlap between sexual dimorphism and FT correlation in PT/PO, plOFC, and RTPJ/pSTS. Red voxels show overlap from the conjunction of FT positive correlation and Male > Female; blue voxels show overlap from the conjunction of FT negative correlation and Female > Male. B, Scatterplot showing the partial correlation between FT and GM volume within sexually dimorphic voxels in right PT/PO. Adjusted predictor and outcome values are plotted on the x and y axes. C, Scatterplot showing the partial correlation between FT and GM volume within sexually dimorphic voxels in RTPJ/pSTS. Adjusted predictor and outcome values are plotted on the x and y axes. D, Scatterplot showing the partial correlation between FT and GM volume within sexually dimorphic voxels in right plOFC. Adjusted predictor and outcome values are plotted on the x and y axes.
Comment in
- Sex steroids and the organization of the human brain.
Peper JS, Koolschijn PC. Peper JS, et al. J Neurosci. 2012 May 16;32(20):6745-6. doi: 10.1523/JNEUROSCI.1012-12.2012. J Neurosci. 2012. PMID: 22593044 Free PMC article. No abstract available.
Similar articles
- Sex steroids and the organization of the human brain.
Peper JS, Koolschijn PC. Peper JS, et al. J Neurosci. 2012 May 16;32(20):6745-6. doi: 10.1523/JNEUROSCI.1012-12.2012. J Neurosci. 2012. PMID: 22593044 Free PMC article. No abstract available. - High fetal testosterone and sexually dimorphic cerebral networks in females.
Ciumas C, Lindén Hirschberg A, Savic I. Ciumas C, et al. Cereb Cortex. 2009 May;19(5):1167-74. doi: 10.1093/cercor/bhn160. Epub 2008 Oct 14. Cereb Cortex. 2009. PMID: 18854582 - Sexual differentiation of the brain: role of testosterone and its active metabolites.
Negri-Cesi P, Colciago A, Celotti F, Motta M. Negri-Cesi P, et al. J Endocrinol Invest. 2004;27(6 Suppl):120-7. J Endocrinol Invest. 2004. PMID: 15481811 Review. - Gonadal steroid action and brain sex differentiation in the rat.
Sakuma Y. Sakuma Y. J Neuroendocrinol. 2009 Mar;21(4):410-4. doi: 10.1111/j.1365-2826.2009.01856.x. J Neuroendocrinol. 2009. PMID: 19226349 Review.
Cited by
- The influence of microsatellite polymorphisms in sex steroid receptor genes ESR1, ESR2 and AR on sex differences in brain structure.
Tan GC, Chu C, Lee YT, Tan CCK, Ashburner J, Wood NW, Frackowiak RS. Tan GC, et al. Neuroimage. 2020 Nov 1;221:117087. doi: 10.1016/j.neuroimage.2020.117087. Epub 2020 Jun 25. Neuroimage. 2020. PMID: 32593802 Free PMC article. - Putative contributions of the sex chromosome proteins SOX3 and SRY to neurodevelopmental disorders.
Tahira AC, Barbosa AR, Feltrin AS, Gastaldi VD, de Toledo VHC, de Carvalho Pereira JG, Lisboa BCG, de Souza Reis VN, Dos Santos ACF, Maschietto M, Brentani H. Tahira AC, et al. Am J Med Genet B Neuropsychiatr Genet. 2019 Sep;180(6):390-414. doi: 10.1002/ajmg.b.32704. Epub 2018 Dec 9. Am J Med Genet B Neuropsychiatr Genet. 2019. PMID: 30537354 Free PMC article. - Association between maternal polycystic ovary syndrome and attention-deficit/hyperactivity disorder in offspring aged 3-6 years: A Chinese population-based study.
Zhang Y, Lu D, Guo VY, Wang Y, Qiu S, Zhang J, Zhang Y, Chen W, Wang B, Yang W. Zhang Y, et al. Front Public Health. 2023 Jan 9;10:1032315. doi: 10.3389/fpubh.2022.1032315. eCollection 2022. Front Public Health. 2023. PMID: 36699874 Free PMC article. - Steroid Metabolites Support Evidence of Autism as a Spectrum.
Gasser BA, Kurz J, Dick B, Mohaupt MG. Gasser BA, et al. Behav Sci (Basel). 2019 May 9;9(5):52. doi: 10.3390/bs9050052. Behav Sci (Basel). 2019. PMID: 31075898 Free PMC article. - Gender-specific differences in the central nervous system's response to anesthesia.
Mawhinney LJ, Mabourakh D, Lewis MC. Mawhinney LJ, et al. Transl Stroke Res. 2013 Aug;4(4):462-75. doi: 10.1007/s12975-012-0229-y. Epub 2012 Nov 29. Transl Stroke Res. 2013. PMID: 24323342 Review.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. - PubMed
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. - PubMed
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. - PubMed
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and autistic traits. Br J Psychol. 2009;100:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01-HD02-3343/HD/NICHD NIH HHS/United States
- N01-NS-9-2319/NS/NINDS NIH HHS/United States
- N01-NS-9-2315/NS/NINDS NIH HHS/United States
- N01 HD023343/HD/NICHD NIH HHS/United States
- G0600977/MRC_/Medical Research Council/United Kingdom
- N01-NS-9-2317/NS/NINDS NIH HHS/United States
- 091774/WT_/Wellcome Trust/United Kingdom
- N01-NS-9-2316/NS/NINDS NIH HHS/United States
- N01-NS-9-2320/NS/NINDS NIH HHS/United States
- N01-MH9-0002/MH/NIMH NIH HHS/United States
- N01-NS-9-2314/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous