Getting in touch with the clathrin terminal domain - PubMed (original) (raw)
Review
Getting in touch with the clathrin terminal domain
Sandra K Lemmon et al. Traffic. 2012 Apr.
Abstract
The N-terminal domain (TD) of the clathrin heavy chain is folded into a seven-bladed β-propeller that projects inward from the polyhedral outer clathrin coat. As the most membrane-proximal portion of assembled clathrin, the TD is a major protein-protein interaction node. Contact with the TD β-propeller occurs through short peptide sequences typically located within intrinsically disordered segments of coat components that usually are elements of the membrane-apposed, inner 'adaptor' coat layer. A huge variation in TD-binding motifs is known and now four spatially discrete interaction surfaces upon the β-propeller have been delineated. An important operational feature of the TD interaction sites in vivo is functional redundancy. The recent discovery that 'pitstop' chemical inhibitors apparently occupy only one of the four TD interaction surfaces, but potently block clathrin-mediated endocytosis, warrants careful consideration of the underlying molecular basis for this inhibition.
© 2012 John Wiley & Sons A/S.
Figures
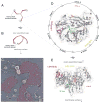
Figure 1. Clathrin structure and function
A. Stylized cartoon depiction of the clathrin triskelion viewed from the ventral surface. The TD at the N-terminal end of each heavy chain (red) is indicated (gray), while the C-terminal end of each heavy chain is involved in trimerization. B. More accurate schematic representation of the lateral view of a clathrin triskelion based on the high-resolution cryoelectron microscopy structure of the assembled cage (63). The arrow indicates the defined sidedness of the assembled trimer. C. Rapid-freeze deep-etch image of assembled clathrin lattice on the glass-adherent ventral surface of a cultured HeLa cell. In these cells, the clathrin polymer (pseudocolored purple) ranges from large expanses of planar assemblies with a preponderance of hexagonal units through adjacent, pentagon-containing hemispherical-shaped buds, to deeply invaginated structures just about to be released from the plasma membrane as clathrin-coated vesicles. The relative positioning of several individual triskelia (red) interdigitated within the assembled lattice is shown. D. Ribbon diagram of the human clathrin TD (PDB code 2XZG (40)) viewed from the membrane-proximal surface of the β-propeller. The positioning of the seven β-stranded blades is indicated as well as the relative locations of the TD interaction surfaces. Rendered in stick representation are some important side chains (with oxygen in red and nitrogen in blue) defining each binding site (Ile80, Gln89, Phe91 and Lys96 for the clathrin-box LØXØ [DE] site 1/purple; Phe27, Gln152, Ile154 and Ile170 for the W-box site 2/green; Arg188 and Gln192 for the [LI][LI]GXL site 3/yellow; Glu11 for site 4/orange). Note the circumferential positioning of the LØXØ [DE], [LI][LI]GXL and site 4 interaction surfaces. E. Ribbon diagram of the TD β-propeller rotated ~90° to illustrate a lateral view. Color-coded as in D with the relative sidedness (arrows) shown.
Similar articles
- Clathrin functions in the absence of the terminal domain binding site for adaptor-associated clathrin-box motifs.
Collette JR, Chi RJ, Boettner DR, Fernandez-Golbano IM, Plemel R, Merz AJ, Geli MI, Traub LM, Lemmon SK. Collette JR, et al. Mol Biol Cell. 2009 Jul;20(14):3401-13. doi: 10.1091/mbc.e08-10-1082. Epub 2009 May 20. Mol Biol Cell. 2009. PMID: 19458198 Free PMC article. - ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2.
He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC. He G, et al. J Biol Chem. 2002 Nov 15;277(46):44044-9. doi: 10.1074/jbc.M208539200. Epub 2002 Sep 8. J Biol Chem. 2002. PMID: 12221107 - Dynamic interactions between clathrin and locally structured elements in a disordered protein mediate clathrin lattice assembly.
Zhuo Y, Ilangovan U, Schirf V, Demeler B, Sousa R, Hinck AP, Lafer EM. Zhuo Y, et al. J Mol Biol. 2010 Nov 26;404(2):274-90. doi: 10.1016/j.jmb.2010.09.044. Epub 2010 Sep 25. J Mol Biol. 2010. PMID: 20875424 Free PMC article. - Clathrin coat construction in endocytosis.
Pearse BM, Smith CJ, Owen DJ. Pearse BM, et al. Curr Opin Struct Biol. 2000 Apr;10(2):220-8. doi: 10.1016/s0959-440x(00)00071-3. Curr Opin Struct Biol. 2000. PMID: 10753805 Review. - Life of a clathrin coat: insights from clathrin and AP structures.
Edeling MA, Smith C, Owen D. Edeling MA, et al. Nat Rev Mol Cell Biol. 2006 Jan;7(1):32-44. doi: 10.1038/nrm1786. Nat Rev Mol Cell Biol. 2006. PMID: 16493411 Review.
Cited by
- Nuclear Magnetic Resonance Structural Mapping Reveals Promiscuous Interactions between Clathrin-Box Motif Sequences and the N-Terminal Domain of the Clathrin Heavy Chain.
Zhuo Y, Cano KE, Wang L, Ilangovan U, Hinck AP, Sousa R, Lafer EM. Zhuo Y, et al. Biochemistry. 2015 Apr 28;54(16):2571-80. doi: 10.1021/acs.biochem.5b00065. Epub 2015 Apr 16. Biochemistry. 2015. PMID: 25844500 Free PMC article. - Clathrin Heavy Chain Interacts With Estrogen Receptor α and Modulates 17β-Estradiol Signaling.
Totta P, Pesiri V, Enari M, Marino M, Acconcia F. Totta P, et al. Mol Endocrinol. 2015 May;29(5):739-55. doi: 10.1210/me.2014-1385. Epub 2015 Apr 10. Mol Endocrinol. 2015. PMID: 25860340 Free PMC article. - Ikarugamycin: A Natural Product Inhibitor of Clathrin-Mediated Endocytosis.
Elkin SR, Oswald NW, Reed DK, Mettlen M, MacMillan JB, Schmid SL. Elkin SR, et al. Traffic. 2016 Oct;17(10):1139-49. doi: 10.1111/tra.12425. Epub 2016 Aug 8. Traffic. 2016. PMID: 27392092 Free PMC article. - Clathrin's adaptor interaction sites are repurposed to stabilize microtubules during mitosis.
Rondelet A, Lin YC, Singh D, Porfetye AT, Thakur HC, Hecker A, Brinkert P, Schmidt N, Bendre S, Müller F, Mazul L, Widlund PO, Bange T, Hiller M, Vetter IR, Bird AW. Rondelet A, et al. J Cell Biol. 2020 Feb 3;219(2):e201907083. doi: 10.1083/jcb.201907083. J Cell Biol. 2020. PMID: 31932847 Free PMC article. - Wbox2: A clathrin terminal domain-derived peptide inhibitor of clathrin-mediated endocytosis.
Chen Z, Mino RE, Mettlen M, Michaely P, Bhave M, Reed DK, Schmid SL. Chen Z, et al. J Cell Biol. 2020 Sep 7;219(9):e201908189. doi: 10.1083/jcb.201908189. J Cell Biol. 2020. PMID: 32520988 Free PMC article.
References
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187–198. - PubMed
- Hughson FM. Copy coats: COPI mimics clathrin and COPII. Cell. 2010;142:19–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM55796/GM/NIGMS NIH HHS/United States
- R01 DK53249/DK/NIDDK NIH HHS/United States
- R01 DK053249/DK/NIDDK NIH HHS/United States
- R01 GM055796/GM/NIGMS NIH HHS/United States
- R01 GM055796-13/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials