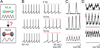Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity - PubMed (original) (raw)
Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity
Court Hull et al. Neuron. 2012.
Abstract
Here we provide evidence that revises the inhibitory circuit diagram of the cerebellar cortex. It was previously thought that Golgi cells, interneurons that are the sole source of inhibition onto granule cells, were exclusively coupled via gap junctions. Moreover, Golgi cells were believed to receive GABAergic inhibition from molecular layer interneurons (MLIs). Here we challenge these views by optogenetically activating the cerebellar circuitry to determine the timing and pharmacology of inhibition onto Golgi cells and by performing paired recordings to directly assess synaptic connectivity. In contrast to current thought, we find that Golgi cells, not MLIs, make inhibitory GABAergic synapses onto other Golgi cells. As a result, MLI feedback does not regulate the Golgi cell network, and Golgi cells are inhibited approximately 2 ms before Purkinje cells, following a mossy fiber input. Hence, Golgi cells and Purkinje cells receive unique sources of inhibition and can differentially process shared granule cell inputs.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
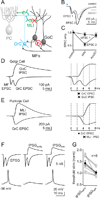
Figure 1. GABAergic inhibitory inputs to Golgi cells
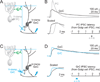
A. Schematic showing the major cell types in the cerebellar cortex with their known synaptic contacts. Abbreviations: PC, Purkinje cell; PF, parallel fiber; MLI, molecular layer interneuron; GrC, granule cell; GoC, Golgi cell; MF, mossy fiber; V-clamp, voltage-clamp. Inhibitory synapses: red minus, and all other synapses (triangles) are glutamatergic. B. Spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from Golgi cells at a holding potential of +10 mV in the presence of NBQX (5 µM) and CPP (2.5 µM) to block glutamatergic inputs. All events were blocked by the GABAAR antagonist gabazine (5 µM). C. Average IPSC evoked with a stimulus electrode placed in the granule cell layer near the recorded Golgi cell. Evoked IPSCs were also blocked by gabazine. D. Fluorescence image of a cerebellar slice from the vermis of a Thy1 ChR2-YFP mouse that expresses channelrhodopsin 2 (ChR2) and YFP in a subset of MFs. GcL = granule cell layer, ML = molecular layer, Scale bar = 100 µm. E. Optical stimulation of ChR2 expressing MFs (473 nm light for 5 ms) evoked EPSCs (recorded at the reversal potential for IPSCs, see methods), and IPSCs (recorded at the EPSC reversal potential). IPSCs evoked by ChR2 activation were blocked by gabazine. F. In another experiment, ChR2-evoked IPSCs were also abolished by blocking glutamatergic transmission (NBQX and CPP), indicating that they were disynaptic.
Figure 2. Timing of MF-evoked inhibition onto Golgi cells, Purkinje cells and granule cells
A. Schematic illustrating the recording configuration for B. Blue bolt represents ChR2 activation with 473 nm light. B. top, Simultaneous recordings from a Purkinje cell (gray) and a Golgi cell (black) at the EPSC reversal potential demonstrate IPSCs onto both cells following ChR2 activation. bottom, left. Scaled IPSCs on an expanded timescale show that the Golgi cell IPSC arrives earlier than the Purkinje cell IPSC. bottom, right. On average, Golgi cell IPSCs arrived nearly 2 ms earlier than IPSCs onto simultaneously recorded Purkinje cells (n=6). C. Schematic of the recording configuration for D. D. top, Simultaneous recordings of IPSCs evoked by ChR2 activation for a granule cell (blue) and a Golgi cell (black). bottom, left. Scaled IPSCs on an expanded timescale show that the Golgi cell IPSC and granule cell IPSC arrive simultaneously. bottom, right. On average, for simultaneously recorded Golgi cells and granule cells, IPSCs arrived synchronously (n=5).
Figure 3. Differential pharmacology IPSCs onto Golgi and Purkinje cells evoked by either MF or PF activation
Following stimulation of either MFs with light (in Thy1 ChR2-YFP mice) (A) or PFs with an extracellular stimulus electrode (B), the resulting disynaptic IPSCs were recorded at Golgi cells and Purkinje cells, and the pharmacological sensitivity to the selective Group II mGluR agonist APDC (2 µM) was measured. As shown in representative experiments for each condition (A, B, left) and in the summaries (A, B, right), Golgi cell IPSCs were strongly attenuated by APDC, but Purkinje cell IPSCs were unaffected. IPSCs in both cells were abolished by blocking glutamatergic transmission (5 µM NBQX and 2.5 µM CPP), indicating that they were disynaptic. Insets show the averaged IPSCs in control (black), APDC (red), washout (blue), and NBQX (green).
Figure 4. Paired recordings reveal that Golgi cells make GABAergic synapses onto each other
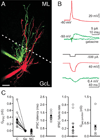
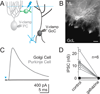
A. A pair of recorded Golgi cells filled with Alexa 488 (green) and Alexa 594 (red), imaged with 2-photon microscopy. Scale bar=20 μm, ML=molecular layer, GcL=granule cell layer, dotted line is the boundary of the molecular layer. B. Experiments to test the electrical and chemical connections between neurons, with traces colored to represent the color of filled neurons in A. top, Spiking one Golgi cell produced an IPSC in the other Golgi cell. The IPSC is the average of 30 consecutive trials, and it was blocked by gabazine. bottom, A current step in one Golgi cell produced a large hyperpolarization in that cell, and a smaller hyperpolarization in the other Golgi cell, indicating that the cells were electrically coupled. C. Summary data from paired recordings (50 directions, 1 pair = 2 tested directions, IPSCs were observed in 10 of 50 directions tested, 3 pairs reciprocally connected). Individual experiments (open circles) and averages (close circles) are shown. left to right: Average conductance, IPSC latency, IPSC failure rate and the mean gap junctional conductance. C = connected, Gz = gabazine, NC = not connected.
Figure 5. Golgi cell spiking is highly sensitive to small, synchronous inhibitory inputs
A. Schematic showing that MLIs are electrically coupled and can fire synchronously, as is the case for Golgi cells. Dynamic clamp experiments were designed to test the implications of weak cross-network synaptic connectivity from MLIs to Golgi cells (B–D). B. Golgi cells were allowed to fire spontaneously at 2 to 8 Hz (B, left) and then inhibitory synaptic conductances (IPSGs, 0.5 – 1 nA) with the time course of inhibitory synaptic currents recorded in Golgi cells (EIPSG = −75 mV) were imposed at 5, 10 and 15 Hz (B, right) to mimic a weak inhibitory input from the MLI network. C. The resulting peristimulus time histograms (PSTH) for the experiment in B shows that inhibition from the MLI network would lead to phase locking of the Golgi cell network. Scale bar (in events/bin/stimulus) top = 0.02 middle = 0.04, bottom = 0.06 D. The average PSTH for 14 experiments also show Golgi cell firing phase locked with its inhibitory inputs. Scale bar (in events/bin/stimulus) top = 0.01 middle = 0.02, bottom = 0.03. Bin widths = 10 ms.
Figure 6. Paired recordings show no connections between molecular layer interneurons and Golgi cells
A. A MLI cell filled with Alexa 488 (green) and a Golgi cell filled with Alexa 594 (red), were imaged with 2-photon microscopy. Scale bar=20 µm, ML=molecular layer, GcL=granule cell layer, dotted line is the boundary of the molecular layer. B. left, Spiking the MLI (green trace) did not produce an IPSC in the Golgi cell (red trace, average of 70 consecutive trials, inflection is a capacitative electrical artifact). right, the average membrane conductance measured from 61 unconnected pairs was 0.001 nS. C. Paired recording from a MLI (basket cell, red) and a Purkinje cell (green). D. left, Spiking the MLI produced an IPSC in the Purkinje cell that was abolished by gabazine (5 µM). right, IPSCs were observed In 6 of 10 paired recordings between MLIs and Purkinje cells. C= connected, Gz = gabazine, NC = not connected.
Figure 7. Transgenic mice expressing ChR2 in MLIs demonstrate a lack of fast inhibitory synapses onto Golgi cells
A. Schematic depicting the paired whole-cell recordings of light-activated currents made simultaneously from a Golgi cell and a Purkinje cell in Prv-mhChR2-YFP mice. B. A fluorescence image shows intense YFP fluorescence in MLIs. Scale bar = 20 µm C. Example of a light-evoked IPSC recorded from a Purkinje cell, with no evoked current in a simultaneously recorded Golgi cell. D. Six such experiments are summarized for Purkinje cells (grey) and Golgi cells (black). No currents were evoked in any Golgi cells, and currents in Purkinje cells (mean conductance = 12.6 nS) were completely blocked by the GABAA receptor antagonist gabazine.
Figure 8. The timing of Golgi cell inhibition matches the timing of granule cell excitation
A. Schematic depicting our revision to the cerebellar circuit diagram. Golgi cells make GABAergic inhibitory synapses onto each other (red circle), and MLIs do not make synapses onto Golgi cells (red X). B. Light activation (0.2 ms, 473 nm) evoked an excitatory current onto a Golgi cell with two distinct components (EPSC1 and EPSC2), in control (black). EPSC2 was reduced in the presence of the type 1 cannabinoid receptor (CB1R) agonist WIN (3 µM; light gray) and recovered by the additional application of the CB1R antagonist AM251 (3 µM; dark gray). C. The effects of the CB1R agonist and antagonist on EPSC1 and EPSC2 are summarized, and each component is normalized to its initial value in control conditions. D. left, Example Golgi cell recording where the same light stimulus produces a dual component EPSC at the IPSC reversal potential, and a disynaptic IPSC at the EPSC reversal potential. right top, The same recording on an expanded time scale shows that the onset of EPSC2 (GrC EPSC) closely matches the onset of the Golgi-cell-mediated IPSC. bottom, On average, the GrC excitation begins at the same time as Golgi-cell-mediated inhibition (delay = 0.1 ms; n =11). E. left, A similar experiment as in D is shown, but while recording from a Purkinje cell. Note that the EPSC onto the Purkinje cell only has one component (from the GrCs). right top, Same recording on an expanded time scale, with the GrC EPSC set to the timing of the GrC EPSC from D. bottom, On average, the GrC excitation precedes the inhibition from MLIs (delay = 1.8 ms; n = 12). F. An example of a dynamic clamp experiment that tests the function of Golgi cell inhibition, and the importance of the timing of this inhibition. The timing and amplitudes of synaptic conductances were based on light-activated responses as in D. Excitatory inputs consisted of an initial mossy fiber component followed by a granule cell component. In the absence of inhibition, this combined input could evoke action potentials for a threshold level stimulus (F, left). In this regime, properly timed inhibition robustly suppressed spiking (F, middle). If the inhibition was delayed, as it would be if it resulted from MLI synapses, the inhibition failed to suppress spiking (F, right). G. Experiments were performed in which the size of the EPSG was varied to determine the threshold for triggering a spike. This was repeated in the presence of properly timed inhibition (IPSGearly) and late inhibition (IPSGlate). The effects of inhibition on threshold are summarized by normalizing to the level of threshold stimulus measured in the absence of inhibition. Individual experiments (open circles) and averages (closed circles) are shown.
Similar articles
- Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration.
Crowley JJ, Fioravante D, Regehr WG. Crowley JJ, et al. Neuron. 2009 Sep 24;63(6):843-53. doi: 10.1016/j.neuron.2009.09.004. Neuron. 2009. PMID: 19778512 Free PMC article. - Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex.
Kanichay RT, Silver RA. Kanichay RT, et al. J Neurosci. 2008 Sep 3;28(36):8955-67. doi: 10.1523/JNEUROSCI.5469-07.2008. J Neurosci. 2008. PMID: 18768689 Free PMC article. - Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex.
Duguid I, Branco T, London M, Chadderton P, Häusser M. Duguid I, et al. J Neurosci. 2012 Aug 8;32(32):11132-43. doi: 10.1523/JNEUROSCI.0460-12.2012. J Neurosci. 2012. PMID: 22875944 Free PMC article. - Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb.
Pressler RT, Strowbridge BW. Pressler RT, et al. Neuron. 2006 Mar 16;49(6):889-904. doi: 10.1016/j.neuron.2006.02.019. Neuron. 2006. PMID: 16543136 - Novel GABAergic circuits mediating excitation/inhibition of Cajal-Retzius cells in the developing hippocampus.
Quattrocolo G, Maccaferri G. Quattrocolo G, et al. J Neurosci. 2013 Mar 27;33(13):5486-98. doi: 10.1523/JNEUROSCI.5680-12.2013. J Neurosci. 2013. PMID: 23536064 Free PMC article.
Cited by
- Multidimensional population activity in an electrically coupled inhibitory circuit in the cerebellar cortex.
Gurnani H, Silver RA. Gurnani H, et al. Neuron. 2021 May 19;109(10):1739-1753.e8. doi: 10.1016/j.neuron.2021.03.027. Epub 2021 Apr 12. Neuron. 2021. PMID: 33848473 Free PMC article. - Cerebellar GABA Change during Visuomotor Adaptation Relates to Adaptation Performance and Cerebellar Network Connectivity: A Magnetic Resonance Spectroscopic Imaging Study.
Nettekoven C, Mitchell L, Clarke WT, Emir U, Campbell J, Johansen-Berg H, Jenkinson N, Stagg CJ. Nettekoven C, et al. J Neurosci. 2022 Oct 12;42(41):7721-7732. doi: 10.1523/JNEUROSCI.0096-22.2022. Epub 2022 Sep 9. J Neurosci. 2022. PMID: 36414012 Free PMC article. - Gap Junctions May Have A Computational Function In The Cerebellum: A Hypothesis.
Gilbert M, Rasmussen A. Gilbert M, et al. Cerebellum. 2024 Oct;23(5):1903-1915. doi: 10.1007/s12311-024-01680-3. Epub 2024 Mar 18. Cerebellum. 2024. PMID: 38499814 Free PMC article. - Gap Junction Modulation of Low-Frequency Oscillations in the Cerebellar Granule Cell Layer.
Robinson JC, Chapman CA, Courtemanche R. Robinson JC, et al. Cerebellum. 2017 Aug;16(4):802-811. doi: 10.1007/s12311-017-0858-5. Cerebellum. 2017. PMID: 28421552 - Cellular-resolution mapping uncovers spatial adaptive filtering at the rat cerebellum input stage.
Casali S, Tognolina M, Gandolfi D, Mapelli J, D'Angelo E. Casali S, et al. Commun Biol. 2020 Oct 30;3(1):635. doi: 10.1038/s42003-020-01360-y. Commun Biol. 2020. PMID: 33128000 Free PMC article.
References
- De Schutter E, Vos B, Maex R. The function of cerebellar Golgi cells revisited. Prog brain res. 2000;124:81–93. - PubMed
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
- Barmack NH, Baughman RW, Eckenstein FP. Cholinergic Innervation of the Cerebellum of Rat, Rabbit, Cat, and Monkey as Activity and Immunohistochemistry. J Comp Neurol. 1992a;317:233–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS032405/NS/NINDS NIH HHS/United States
- R37 NS032405/NS/NINDS NIH HHS/United States
- R37 NS032405-17/NS/NINDS NIH HHS/United States
- F32 NS060585/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous