Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts - PubMed (original) (raw)
Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts
Narutoshi Hibino et al. J Thorac Cardiovasc Surg. 2012 Mar.
Abstract
Objective: The development of a living, tissue-engineered vascular graft (TEVG) holds great promise for advancing the field of cardiovascular surgery. However, the ultimate source and time needed to procure these cells remain problematic. Induced puripotent stem (iPS) cells have recently been developed and have the potential for creating a pluripotent cell line from a patient's own somatic cells. In the present study, we evaluated the use of a sheet created from iPS cell-derived vascular cells as a potential source for the construction of TEVG.
Methods: Male mouse iPS cells were differentiated into embryoid bodies using the hanging-drop method. Cell differentiation was confirmed by a decrease in the proportion of SSEA-1-positive cells over time using fluorescence-activated cell sorting. The expression of endothelial cell and smooth muscle cell markers was detected using real-time polymerase chain reaction (PCR). The differentiated iPS cell sheet was made using temperature-responsive dishes and then seeded onto a biodegradable scaffold composed of polyglycolic acid-poly-l-lactide and poly(l-lactide-co-ε-caprolactone) with a diameter of 0.8 mm. These scaffolds were implanted as interposition grafts in the inferior vena cava of female severe combined immunodeficiency/beige mice (n = 15). Graft function was serially monitored using ultrasonography. The grafts were analyzed at 1, 4, and 10 weeks with histologic examination and immunohistochemistry. The behavior of seeded differentiated iPS cells was tracked using Y-chromosome fluorescent in situ hybridization and SRY real-time PCR.
Results: All mice survived without thrombosis, aneurysm formation, graft rupture, or calcification. PCR evaluation of iPS cell sheets in vitro demonstrated increased expression of endothelial cell markers. Histologic evaluation of the grafts demonstrated endothelialization with von Willebrand factor and an inner layer with smooth muscle actin- and calponin-positive cells at 10 weeks. The number of seeded differentiated iPS cells was found to decrease over time using real-time PCR (42.2% at 1 week, 10.4% at 4 weeks, 9.8% at 10 weeks). A fraction of the iPS cells were found to be Y-chromosome fluorescent positive at 1 week. No iPS cells were found to co-localize with von Willebrand factor or smooth muscle actin-positive cells at 10 weeks.
Conclusions: Differentiated iPS cells offer an alternative cell source for constructing TEVG. Seeded iPS cells exerted a paracrine effect to induce neotissue formation in the acute phase and were reduced in number by apoptosis at later time points. Sheet seeding of our TEVG represents a viable mode of iPS cell delivery over time.
Copyright © 2012. Published by Mosby, Inc.
Figures
Figure 1
Confirmation of differentiation of iPS cells in vitro. (a) The ratio of undifferentiated SSEA-1 positive cells by FACS analysis decreased over time after changing to differentiation medium. (b) Expression of endothelial markers (VEGF, PECAM and E-cadherin) and smooth muscle cell marker (calponin) in differentiated iPS cells.
Figure 2
Attached cell number and seeding efficiency of differentiated iPS cells seeded onto the scaffold. There was no significant difference for any incubation period.
Figure 3
Differentiated iPS cell sheet seeded TEVG. (a) Cell sheet creation from differentiated iPS cells. (b) The scaffold was wrapped with this cell sheet for seeding. (c) New seeding method using a differentiated iPS cell sheet significantly improved seeding efficiency compared with traditional pipette seeding. (d) Differentiated iPS cell sheet seeded TEVG was implanted in mouse IVC.
Figure 4
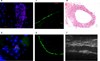
Histology of TEVG. (a) Seeded iPS cells were identified as Y chromosome FISH positive cells (red) at 1 week following implantation (Blue=DAPI). (b) At 4 weeks very small numbers of seeded cells were found. (c, d) At 10weeks, there was endothelialization with VWF (green) (c) and an inner layer with SMA (green) (d) positive cells at 10 weeks; however, no Y chromosome FISH positive seeded iPS cells (red) were seen. H&E staining (e) and ultrasonography (f) showed patent graft at 10 weeks.
Figure 5
Quantification of seeded differentiated iPS cells by quantitative real time PCR using probe for SRY. The number of seeded iPS cells decreased dramatically over time.
Figure 6
There was mixture of surviving (yellow arrows) and apoptotic (white arrows) seeded iPS cells at 1 week after graft implantation. (Green= TUNEL positive, Red= SRY FISH positive, Blue= DAPI
Similar articles
- Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft.
Tara S, Kurobe H, Maxfield MW, Rocco KA, Yi T, Naito Y, Breuer CK, Shinoka T. Tara S, et al. J Vasc Surg. 2015 Sep;62(3):734-43. doi: 10.1016/j.jvs.2014.03.011. Epub 2014 Apr 16. J Vasc Surg. 2015. PMID: 24745941 - Tissue-engineered Vascular Grafts in Children With Congenital Heart Disease: Intermediate Term Follow-up.
Sugiura T, Matsumura G, Miyamoto S, Miyachi H, Breuer CK, Shinoka T. Sugiura T, et al. Semin Thorac Cardiovasc Surg. 2018 Summer;30(2):175-179. doi: 10.1053/j.semtcvs.2018.02.002. Epub 2018 Feb 7. Semin Thorac Cardiovasc Surg. 2018. PMID: 29427773 Free PMC article. Clinical Trial. - Role of Bone Marrow Mononuclear Cell Seeding for Nanofiber Vascular Grafts.
Fukunishi T, Best CA, Ong CS, Groehl T, Reinhardt J, Yi T, Miyachi H, Zhang H, Shinoka T, Breuer CK, Johnson J, Hibino N. Fukunishi T, et al. Tissue Eng Part A. 2018 Jan;24(1-2):135-144. doi: 10.1089/ten.TEA.2017.0044. Epub 2017 Jun 13. Tissue Eng Part A. 2018. PMID: 28486019 Free PMC article. - Vascular tissue engineering: towards the next generation vascular grafts.
Naito Y, Shinoka T, Duncan D, Hibino N, Solomon D, Cleary M, Rathore A, Fein C, Church S, Breuer C. Naito Y, et al. Adv Drug Deliv Rev. 2011 Apr 30;63(4-5):312-23. doi: 10.1016/j.addr.2011.03.001. Epub 2011 Mar 21. Adv Drug Deliv Rev. 2011. PMID: 21421015 Review. - In vivo applications of electrospun tissue-engineered vascular grafts: a review.
Rocco KA, Maxfield MW, Best CA, Dean EW, Breuer CK. Rocco KA, et al. Tissue Eng Part B Rev. 2014 Dec;20(6):628-40. doi: 10.1089/ten.TEB.2014.0123. Epub 2014 Jun 18. Tissue Eng Part B Rev. 2014. PMID: 24786567 Review.
Cited by
- Differential outcomes of venous and arterial tissue engineered vascular grafts highlight the importance of coupling long-term implantation studies with computational modeling.
Best CA, Szafron JM, Rocco KA, Zbinden J, Dean EW, Maxfield MW, Kurobe H, Tara S, Bagi PS, Udelsman BV, Khosravi R, Yi T, Shinoka T, Humphrey JD, Breuer CK. Best CA, et al. Acta Biomater. 2019 Aug;94:183-194. doi: 10.1016/j.actbio.2019.05.063. Epub 2019 Jun 12. Acta Biomater. 2019. PMID: 31200116 Free PMC article. - Engineered Microenvironment for Manufacturing Human Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells.
Lin H, Qiu X, Du Q, Li Q, Wang O, Akert L, Wang Z, Anderson D, Liu K, Gu L, Zhang C, Lei Y. Lin H, et al. Stem Cell Reports. 2019 Jan 8;12(1):84-97. doi: 10.1016/j.stemcr.2018.11.009. Epub 2018 Dec 6. Stem Cell Reports. 2019. PMID: 30527760 Free PMC article. - Stem Cell Sources and Graft Material for Vascular Tissue Engineering.
Hielscher D, Kaebisch C, Braun BJV, Gray K, Tobiasch E. Hielscher D, et al. Stem Cell Rev Rep. 2018 Oct;14(5):642-667. doi: 10.1007/s12015-018-9825-x. Stem Cell Rev Rep. 2018. PMID: 29860617 Review. No abstract available. - Human Peripheral Blood-Derived Endothelial Colony-Forming Cells Are Highly Similar to Mature Vascular Endothelial Cells yet Demonstrate a Transitional Transcriptomic Signature.
Kutikhin AG, Tupikin AE, Matveeva VG, Shishkova DK, Antonova LV, Kabilov MR, Velikanova EA. Kutikhin AG, et al. Cells. 2020 Apr 3;9(4):876. doi: 10.3390/cells9040876. Cells. 2020. PMID: 32260159 Free PMC article. - Cellularized small-caliber tissue-engineered vascular grafts: looking for the ultimate gold standard.
Fayon A, Menu P, El Omar R. Fayon A, et al. NPJ Regen Med. 2021 Aug 12;6(1):46. doi: 10.1038/s41536-021-00155-x. NPJ Regen Med. 2021. PMID: 34385472 Free PMC article. Review.
References
- Goyal A, Wang Y, Su H, Dobrucki LW, Brennan M, Fong P, et al. Development of a model system for preliminary evaluation of tissue-engineered vascular conduits. J Pediatr Surg. 2006 Apr;41(4):787–791. - PubMed
- Hibino N, Shin'oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005 May;129(5):1064–1070. - PubMed
- Shinoka T, Shum-Tim D, Ma PX, Tanel RE, Isogai N, Langer R, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998 Mar;115(3):536–545. discussion 45-6. - PubMed
- Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006 Nov;12(11):3075–3083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 HL101990-03/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K02 HL101990/HL/NHLBI NIH HHS/United States
- R01 HL069368/HL/NHLBI NIH HHS/United States
- K02 HL101990-01/HL/NHLBI NIH HHS/United States
- K02 HL101990-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources