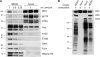Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane - PubMed (original) (raw)
Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane
Mathias J Gerl et al. J Cell Biol. 2012.
Abstract
The influenza virus (IFV) acquires its envelope by budding from host cell plasma membranes. Using quantitative shotgun mass spectrometry, we determined the lipidomes of the host Madin-Darby canine kidney cell, its apical membrane, and the IFV budding from it. We found the apical membrane to be enriched in sphingolipids (SPs) and cholesterol, whereas glycerophospholipids were reduced, and storage lipids were depleted compared with the whole-cell membranes. The virus membrane exhibited a further enrichment of SPs and cholesterol compared with the donor membrane at the expense of phosphatidylcholines. Our data are consistent with and extend existing models of membrane raft-based biogenesis of the apical membrane and IFV envelope.
Figures
Figure 1.
Biochemical analysis of the AP. (A) Western blot analysis of apical membrane proteins (Apical) that were recovered from NC membranes peeled from polarized MDCK cells. Total cells (MDCK) serve as a reference. Podocalyxin (PDX), gp114, and syntaxin 3 (STX3) were used as markers for the apical membrane, Occludin (Occ) was used for tight junctions, integrin β1 (Int β1)/E-cadherin (E-cad) were used for the basolateral membrane, calnexin (CNX) was used for the ER, furin was used for the TGN, and histone H3 was used for the nucleus. Samples were standardized to total protein amount. (B) Polarized MDCK cells were either biotinylated apically (a) or basolaterally (b). Apical membrane proteins (Apical) and total cells (MDCK) were prepared as in A. The degree of biotinylation was visualized by streptavidin-HRP. rel. amount, relative amount. Molecular markers are given in kilodaltons.
Figure 2.
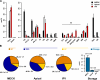
Lipid class composition and functional categories. (A and B) The content of individual lipid classes (A) and functional lipid categories (B) of total cells (MDCK), the AP (Apical), and influenza virus (IFV) was determined by summing up absolute abundances of all identified species. Values are standardized to mole percentage of all membrane lipids within the sample without storage lipids. Indicated values have been multiplied by five for visibility. Sterols (ST): cholesterol (Chol) and desmosterol (Desm). GPLs: DAG, phosphatidic acid (PA), phosphatidylcholine/-ethanolamine/-glycerol/-inositol/-serine (PC/PE/PG/PI/PS), and ether linked PC/PE (PC O-/PE O-). Sphingolipids (SP): ceramide (Cer), Forssman glycolipid (For), hexosylceramide (HexCer), dihexosylceramide ((Hex)2Cer), sphingomyelin (SM), and sulfatide (Sulf). Storage: cholesterol esters (CE) and triacylglycerol (TAG). Error bars correspond to SDs (n = 3).
Figure 3.
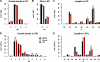
Lipid features of total cells, the apical membrane, and IFV. (A–E) Changes in SP saturation as number of double bonds (db) in the sum of long chain base and fatty acid (A), SP hydroxylation (B), SP chain length as number of carbon atoms in the sum of long chain base and fatty acid (C), GPL saturation as number of double bonds in the sum of fatty acid moieties (D), and GPL chain length as number of carbon atoms in the sum of fatty acid moieties (E). Values are standardized to mole percentage of all GPLs or SPs as indicated. Features were calculated from individual quantities of lipid molecular species. Error bars correspond to SDs (n = 3).
Figure 4.
IFV purification. Polarized MDCK cells were infected with IFV, and virus particles were collected after 24 h. (A) A pure virus preparation was obtained by gradient centrifugations and was analyzed by SDS-PAGE and silver staining. (B) Electron micrograph of the virus preparation with negative stain. The inset shows a higher magnification of the same preparation. Molecular markers are given in kilodaltons. NP, influenza nucleoprotein.
Figure 5.
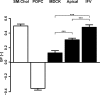
Membrane order measurements by C-laurdan spectroscopy. Generalized polarization (GP) values of liposomes with defined liquid-ordered (SM/cholesterol [Chol] = 1:1) and liquid-disordered (POPC) compositions (open bars) compared with GP values of floated total MDCK membranes (MDCK), floated apical membranes (Apical), and influenza virus (IFV; shaded bars). Measurements were performed at 23°C. The p-values have been estimated with one-way analysis of variance and posthoc test with Bonferroni correction (***, P < 0.001). Error bars correspond to SDs (n = 3).
Similar articles
- Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells.
van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. van Meer G, et al. J Cell Biol. 1987 Oct;105(4):1623-35. doi: 10.1083/jcb.105.4.1623. J Cell Biol. 1987. PMID: 3667693 Free PMC article. - Cholesterol is enriched in the sphingolipid patches on the substrate near nonpolarized MDCK cells, but not in the sphingolipid domains in their plasma membranes.
Yeager AN, Weber PK, Kraft ML. Yeager AN, et al. Biochim Biophys Acta Biomembr. 2018 Oct;1860(10):2004-2011. doi: 10.1016/j.bbamem.2018.04.008. Epub 2018 Apr 22. Biochim Biophys Acta Biomembr. 2018. PMID: 29684331 - Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells.
Rindler MJ, Ivanov IE, Plesken H, Rodriguez-Boulan E, Sabatini DD. Rindler MJ, et al. J Cell Biol. 1984 Apr;98(4):1304-19. doi: 10.1083/jcb.98.4.1304. J Cell Biol. 1984. PMID: 6325468 Free PMC article. - Structure and function of sphingolipid- and cholesterol-rich membrane rafts.
Brown DA, London E. Brown DA, et al. J Biol Chem. 2000 Jun 9;275(23):17221-4. doi: 10.1074/jbc.R000005200. J Biol Chem. 2000. PMID: 10770957 Review. No abstract available. - Lipid polarity and sorting in epithelial cells.
van Meer G, Simons K. van Meer G, et al. J Cell Biochem. 1988 Jan;36(1):51-8. doi: 10.1002/jcb.240360106. J Cell Biochem. 1988. PMID: 3277985 Review.
Cited by
- The amphipathic helix of influenza A virus M2 protein is required for filamentous bud formation and scission of filamentous and spherical particles.
Roberts KL, Leser GP, Ma C, Lamb RA. Roberts KL, et al. J Virol. 2013 Sep;87(18):9973-82. doi: 10.1128/JVI.01363-13. Epub 2013 Jul 10. J Virol. 2013. PMID: 23843641 Free PMC article. - Characterization of the Lipidome and Biophysical Properties of Membranes from High Five Insect Cells Expressing Mouse P-Glycoprotein.
Moreno MJ, Teles Martins PA, Bernardino EF, Abel B, Ambudkar SV. Moreno MJ, et al. Biomolecules. 2021 Mar 14;11(3):426. doi: 10.3390/biom11030426. Biomolecules. 2021. PMID: 33799403 Free PMC article. - Hemagglutinin-esterase-fusion (HEF) protein of influenza C virus.
Wang M, Veit M. Wang M, et al. Protein Cell. 2016 Jan;7(1):28-45. doi: 10.1007/s13238-015-0193-x. Epub 2015 Jul 28. Protein Cell. 2016. PMID: 26215728 Free PMC article. Review. - Sphingolipids as Potential Therapeutic Targets against Enveloped Human RNA Viruses.
Yager EJ, Konan KV. Yager EJ, et al. Viruses. 2019 Oct 1;11(10):912. doi: 10.3390/v11100912. Viruses. 2019. PMID: 31581580 Free PMC article. Review. - Annexin A6-balanced late endosomal cholesterol controls influenza A replication and propagation.
Musiol A, Gran S, Ehrhardt C, Ludwig S, Grewal T, Gerke V, Rescher U. Musiol A, et al. mBio. 2013 Nov 5;4(6):e00608-13. doi: 10.1128/mBio.00608-13. mBio. 2013. PMID: 24194536 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous