Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs - PubMed (original) (raw)
Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration-approved drugs
B L Ellis et al. Gene Ther. 2013 Jan.
Abstract
An emerging strategy for the treatment of monogenic diseases uses genetic engineering to precisely correct the mutation(s) at the genome level. Recent advancements in this technology have demonstrated therapeutic levels of gene correction using a zinc-finger nuclease (ZFN)-induced DNA double-strand break in conjunction with an exogenous DNA donor substrate. This strategy requires efficient nucleic acid delivery and among viral vectors, recombinant adeno-associated virus (rAAV) has demonstrated clinical success without pathology. However, a major limitation of rAAV is the small DNA packaging capacity and to date, the use of rAAV for ZFN gene delivery has yet to be reported. Theoretically, an ideal situation is to deliver both ZFNs and the repair substrate in a single vector to avoid inefficient gene targeting and unwanted mutagenesis, both complications of a rAAV co-transduction strategy. Therefore, a rAAV format was generated in which a single polypeptide encodes the ZFN monomers connected by a ribosome skipping 2A peptide and furin cleavage sequence. On the basis of this arrangement, a DNA repair substrate of 750 nucleotides was also included in this vector. Efficient polypeptide processing to discrete ZFNs is demonstrated, as well as the ability of this single vector format to stimulate efficient gene targeting in a human cell line and mouse model derived fibroblasts. Additionally, we increased rAAV-mediated gene correction up to sixfold using a combination of Food and Drug Administration-approved drugs, which act at the level of AAV vector transduction. Collectively, these experiments demonstrate the ability to deliver ZFNs and a repair substrate by a single AAV vector and offer insights for the optimization of rAAV-mediated gene correction using drug therapy.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
Figure 1
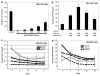
Schematic representations of the genomic contents of AAV6 ZFN2/1/D and AAV6 donor. (a) The genome of the AAV6 viral vector ZFN2/1/D, which contains DNA encoding two GFP ZFNs separated by a 2A peptide driven by the ubiquitin C promoter, and includes the eGFP gene truncated at nucleotide 37 as a donor substrate in gene targeting by homologous recombination. (b) The genome of the AAV6 viral vector donor, which contains the same truncated, non-functional form of eGFP as in (a), and is driven by the cytomegalovirus promoter. (c) Western blot from calcium phosphate transfection of ZFN2/1/D construct in the AAV backbone (lane 1), the ZFN2/1/donor* construct in a lentiviral backbone (lane 2), the ZFN2 construct in a lentiviral backbone (lane 3) and the ZFN1 construct in a lentiviral backbone (lane 4). Note that most of the protein from lane 1 is present at the monomeric weight (37 kD marker).
Figure 2
ZFN-mediated gene targeting in HEK 293 cells delivered by AAV6. (a) Gene targeting in HEK 293 cells with increasing MOIs of AAV6 ZFN 2/1/D virus, analyzed on day 3 for GFP by flow cytometry. (b) Gene targeting in HEK 293 cells with increasing MOIs of AAV6 donor virus and a constant MOI of 300K for the AAV6 ZFN 2/1/D virus, analyzed on day 3 for GFP by flow cytometry. (c) Kinetic analysis of gene targeting in HEK 293 cells with increasing MOIs of AAV6 ZFN 2/1/D virus analyzed by flow cytometry at the indicated time points post infection. (d) Gene targeting in HEK 293 cells with increasing MOIs of AAV6 donor virus and a constant MOI of 300K for the AAV6 ZFN 2/1/D virus, analyzed at the indicated time points for GFP by flow cytometry. *Significantly different compared with the lipofected sample. n = 3, P < 0.05 (for c and d: only evaluated at the last time point between lipofection and the infected population closest to the lipofected value).
Figure 3
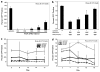
ZFN-mediated gene targeting in Rosa 26 3T3 cells delivered by rAAV6. (a) Gene targeting in Rosa 26 3T3 cells with increasing MOIs of rAAV6 ZFN 2/1/D virus, analyzed on day 3 for GFP by flow cytometry. (b) Gene targeting in Rosa 26 3T3 cells with increasing MOIs of rAAV6 donor virus and a constant MOI of 300K for the rAAV6 ZFN 2/1/D virus, analyzed on day 3 for GFP by flow cytometry. (c) Kinetic analysis of gene targeting in Rosa 26 3T3 cells with increasing MOIs of rAAV6 ZFN 2/1/D virus, analyzed for GFP by flow cytometry. (d) Kinetic analysis of gene targeting in Rosa 26 3T3 cells with increasing MOIs of rAAV6 donor virus and a constant MOI of 300K for the rAAV6 ZFN 2/1/D virus. GFP-positive cells were then analyzed at the indicated time points by flow cytometry.*Significantly different compared with the lipofected sample. n = 3, P < 0.05 (for c and d: only evaluated at the last time point between lipofection and the infected population closest to the lipofected value).
Figure 4
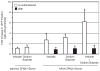
Enhancement of gene targeting using Food and Drug Administration-approved drugs. HEK 293 cells were transduced with rAAV6 ZFN 2/1/donor at 100 000 viral genomes per cell. At the time of vector addition (co-administered) or 18 h post vector addition, cells were treated with the indicated drug (bortezomib 2 μM; sodium butyrate 1.5 μM). Two days following vector addition, cells were harvested and GFP +cells were quantitated by flow cytometry. The value determined in the presence of the drug was divided by the value determined in the absence of the drug and the data are presented as a fold change. Plasmid experiments relied on the same general strategy, however, in these cases the drug was given 4 h pos-transfection.
Similar articles
- The nontoxic cell cycle modulator indirubin augments transduction of adeno-associated viral vectors and zinc-finger nuclease-mediated gene targeting.
Rahman SH, Bobis-Wozowicz S, Chatterjee D, Gellhaus K, Pars K, Heilbronn R, Jacobs R, Cathomen T. Rahman SH, et al. Hum Gene Ther. 2013 Jan;24(1):67-77. doi: 10.1089/hum.2012.168. Epub 2012 Dec 10. Hum Gene Ther. 2013. PMID: 23072634 Free PMC article. - Versatile and efficient genome editing in human cells by combining zinc-finger nucleases with adeno-associated viral vectors.
Händel EM, Gellhaus K, Khan K, Bednarski C, Cornu TI, Müller-Lerch F, Kotin RM, Heilbronn R, Cathomen T. Händel EM, et al. Hum Gene Ther. 2012 Mar;23(3):321-9. doi: 10.1089/hum.2011.140. Epub 2011 Dec 14. Hum Gene Ther. 2012. PMID: 21980922 Free PMC article. - Delivering Transgenic DNA Exceeding the Carrying Capacity of AAV Vectors.
Hirsch ML, Wolf SJ, Samulski RJ. Hirsch ML, et al. Methods Mol Biol. 2016;1382:21-39. doi: 10.1007/978-1-4939-3271-9_2. Methods Mol Biol. 2016. PMID: 26611576 Free PMC article. - AAV Vectorization of DSB-mediated Gene Editing Technologies.
Moser RJ, Hirsch ML. Moser RJ, et al. Curr Gene Ther. 2016;16(3):207-19. doi: 10.2174/1566523216666160602213738. Curr Gene Ther. 2016. PMID: 27280971 Review. - Understanding AAV vector immunogenicity: from particle to patient.
Dhungel BP, Winburn I, Pereira CDF, Huang K, Chhabra A, Rasko JEJ. Dhungel BP, et al. Theranostics. 2024 Jan 20;14(3):1260-1288. doi: 10.7150/thno.89380. eCollection 2024. Theranostics. 2024. PMID: 38323309 Free PMC article. Review.
Cited by
- The nontoxic cell cycle modulator indirubin augments transduction of adeno-associated viral vectors and zinc-finger nuclease-mediated gene targeting.
Rahman SH, Bobis-Wozowicz S, Chatterjee D, Gellhaus K, Pars K, Heilbronn R, Jacobs R, Cathomen T. Rahman SH, et al. Hum Gene Ther. 2013 Jan;24(1):67-77. doi: 10.1089/hum.2012.168. Epub 2012 Dec 10. Hum Gene Ther. 2013. PMID: 23072634 Free PMC article. - In Vivo Delivery Systems for Therapeutic Genome Editing.
Wang L, Li F, Dang L, Liang C, Wang C, He B, Liu J, Li D, Wu X, Xu X, Lu A, Zhang G. Wang L, et al. Int J Mol Sci. 2016 Apr 27;17(5):626. doi: 10.3390/ijms17050626. Int J Mol Sci. 2016. PMID: 27128905 Free PMC article. Review. - Targeted Gene Insertion for Functional CFTR Restoration in Airway Epithelium.
Barillà C, Suzuki S, Rab A, Sorscher EJ, Davis BR. Barillà C, et al. Front Genome Ed. 2022 Mar 7;4:847645. doi: 10.3389/fgeed.2022.847645. eCollection 2022. Front Genome Ed. 2022. PMID: 35330693 Free PMC article. - Adenoviral vector DNA for accurate genome editing with engineered nucleases.
Holkers M, Maggio I, Henriques SF, Janssen JM, Cathomen T, Gonçalves MA. Holkers M, et al. Nat Methods. 2014 Oct;11(10):1051-7. doi: 10.1038/nmeth.3075. Epub 2014 Aug 24. Nat Methods. 2014. PMID: 25152084 - CRISPR/Cas: a Nobel Prize award-winning precise genome editing technology for gene therapy and crop improvement.
Li C, Brant E, Budak H, Zhang B. Li C, et al. J Zhejiang Univ Sci B. 2021 Apr 15;22(4):253-284. doi: 10.1631/jzus.B2100009. J Zhejiang Univ Sci B. 2021. PMID: 33835761 Free PMC article. Review.
References
- Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. 20 years of gene therapy for SCID. Nat Immunol. 2010;11:457– 460. - PubMed
- Cappelli B, Aiuti A. Gene therapy for adenosine deaminase deficiency. Immunol Allergy Clin North Am. 2010;30:249– 260. - PubMed
- Qasim W, Gaspar HB, Thrasher AJ. Progress and prospects: gene therapy for inherited immunodeficiencies. Gene Therapy. 2009;16:1285– 1291. - PubMed
- Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI072176/AI/NIAID NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- PN2EY018244/EY/NEI NIH HHS/United States
- PN2 EY018244/EY/NEI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources