EphA2 receptor activation by monomeric Ephrin-A1 on supported membranes - PubMed (original) (raw)
EphA2 receptor activation by monomeric Ephrin-A1 on supported membranes
Qian Xu et al. Biophys J. 2011.
Abstract
The receptor tyrosine kinase EphA2 interacts with its glycosylphosphatidylinositol (GPI)-linked ephrin-A1 ligand in a juxtacrine configuration. The soluble ephrin-A1 protein, without its GPI membrane linker, fails to activate EphA2. However, preclustered ephrin-A1 protein is active in solution and has been frequently used to trigger the EphA2 receptor. Although this approach has yielded insights into EphA2 signaling, preclustered ligands bypass natural receptor clustering processes and thus mask any role of clustering as a signal regulatory mechanism. Here, we present EphA2-expressing cells with a fusion protein of monomeric ephrin-A1 (mEA1) and enhanced monomeric yellow fluorescent protein that is linked to a supported lipid bilayer via a nickel-decahistidine anchor. The mEA1 is homogeneously dispersed, laterally mobile, and monomeric as measured by fluorescence imaging, correlation spectroscopy, and photon counting histogram analysis, respectively. Ephrin-A1 presented in this manner activates EphA2 on the surface of MDA-MB-231 human breast cancer cells, as measured by EphA2 phosphorylation and degradation. Spatial mutation experiments in which nanopatterns on the underlying substrate restrict mEA1 movement in the supported lipid bilayer reveal spatio-mechanical regulation of this signaling pathway, consistent with recently reported observations using a synthetically cross-linked ephrin-A1 dimer.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
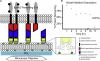
Figure 1
Schematic diagram of the experimental setup. (A) EphA2-expressing breast cancer cells are cultured on a SLB consisting of a tunable surface density of EA1 fusion proteins. This fusion protein is designed by linking the soluble portion of monomeric human ephrin-A1 with enhanced YFP that has an A206K mutation to prevent dimer formation. The inset shows the anchoring strategy, which is stable when the decahistidine sequence at the C-terminus of the fusion protein is chelating multiple Ni ions that are chelated by a tunable subset of lipids within the bilayer. (B) Ni-histidine dissociation curve shows that protein binding reaches a kinetically trapped state (plateau in graph between 3 and 16 h) that is stable and therefore insensitive to rinsing steps well beyond the timescale of experiments. The bilayer is incubated at 25°C for the entirety of the measurements except for an hour at 37°C in warm cell media after the first 2 h. The high temperature incubation period is performed to mimic the period after cells are introduced and then incubated at 37°C for an hour.
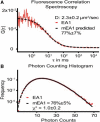
Figure 2
Characterization of mEA1-SLB surface heterogeneity and protein cluster size. (A) FCS is used to determine the heterogeneity of the surface. Values are fitted to a standard two-dimensional, single component curve. The diffusion constant calculated for the majority species is comparable to protein diffusion constants on cell membranes in vivo. A derivation of the autocorrelation function is used to relate the fraction of monomer to the average aggregation number (Q). From two independent FCS experiments, the derived function predicts that the fraction of monomeric species is 77 ± 7% for a Q value of three. (B) The PCH is best fit by a two species fit. The majority of the surface protein molecules (76 ± 5%) exhibit an average fluorescence intensity corresponding to a single EYFP flourophore, indicating that the majority of the species exists as monomer fusion proteins. This percentage is an average across three independent PCH experiments.
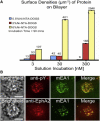
Figure 3
Surface density titration of mEA1 and immunofluorescence images of MDA-MB-231 cells. (A) The surface density of mEA1 is achieved by varying the solution incubation concentration above the bilayer and the molar ratio of Ni capturing lipids within the bilayer through kinetic control parameters. The surface density measurements are performed using quantitative fluorescence microscopy. (B) MDA-MB-231 cells are fixed and permeabilized after 15-min incubations on these surfaces. Antibodies against phosphorylated tyrosine residues (pY) and EphA2 are used to detect phosphorylation at the regions of mEA1 cluster formation and to stain for the presence of EphA2, respectively. For high mEA1 surface densities (thousands of molecules/_μ_m2), phosphorylated proteins are recruited to the mEA1 microclusters. At low mEA1 surface densities (hundreds of molecules/_μ_m2), EphA2 is also recruited. These results suggest that over a range of surface densities, phosphorylated proteins and EphA2 colocalize with mEA1 on the single cell level. A similar result is observed for EphA2 at high mEA1 surface densities and pY at low mEA1 surface densities; recruitment of both molecules occurs over a range of mEA1 surface densities (results not shown). Scale bars are 10 _μ_m.
Figure 4
Western blots are analyzed from the lysate of MDA-MB-231 cells incubated on different surfaces and in different solutions. The blots are stained for the presence of EphA2. In this case, the degradation of EphA2 is represented by the intensity of an EphA2 band between 75 and 150 kDa. The lower the band intensity, the greater the receptor degradation. Intensity measurements of EphA2 bands are repeated for at least four unique Western blots and the results are averaged across the blots. Soluble mEA1, over a range of concentrations (results not shown), does not induce significant EphA2 degradation, whereas mEA1 on a SLB leads to EphA2 degradation. Antibody cross-linked mEA1 on a SLB leads to EphA2 degradation, although to a lesser degree than preclustered EA1-Fc. At low surface densities of mEA1, Western blot analysis is unable to detect significant EphA2 activation (Figs. S8–S10).
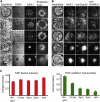
Figure 5
EphA2 pathway activated by mEA1 shows a spatio-mechanical regulatory component. (A) Ligand-induced EphA2 clustering is restricted with chromium barriers. Epifluorescence images show cytoskeleton annulus formation when transport is unrestricted and cytoskeleton spreading morphology when transport is restricted. (B) Total internal reflection fluorescence microscopy and (C) subsequent quantitative colocalization analysis of EphA2 to ADAM10 reveals that ADAM10 recruitment occurs only when receptor transport is unhindered. An average of 200 cells was analyzed for each grid pitch. The surface density of mEA1 used for these experiments is approximately hundreds of molecules/_μ_m2. Scale bars are 10 _μ_m.
Similar articles
- Restriction of receptor movement alters cellular response: physical force sensing by EphA2.
Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Salaita K, et al. Science. 2010 Mar 12;327(5971):1380-5. doi: 10.1126/science.1181729. Science. 2010. PMID: 20223987 Free PMC article. - Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase.
Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W. Ferluga S, et al. J Biol Chem. 2013 Jun 21;288(25):18448-57. doi: 10.1074/jbc.M113.464008. Epub 2013 May 9. J Biol Chem. 2013. PMID: 23661698 Free PMC article. - EphA2 receptor activation with ephrin-A1 ligand restores cetuximab efficacy in NRAS-mutant colorectal cancer cells.
Cuyàs E, Queralt B, Martin-Castillo B, Bosch-Barrera J, Menendez JA. Cuyàs E, et al. Oncol Rep. 2017 Jul;38(1):263-270. doi: 10.3892/or.2017.5682. Epub 2017 May 30. Oncol Rep. 2017. PMID: 28560458 - Ephs and ephrins in cancer: ephrin-A1 signalling.
Beauchamp A, Debinski W. Beauchamp A, et al. Semin Cell Dev Biol. 2012 Feb;23(1):109-15. doi: 10.1016/j.semcdb.2011.10.019. Epub 2011 Oct 25. Semin Cell Dev Biol. 2012. PMID: 22040911 Free PMC article. Review. - Roles of EphA1/A2 and ephrin-A1 in cancer.
Ieguchi K, Maru Y. Ieguchi K, et al. Cancer Sci. 2019 Mar;110(3):841-848. doi: 10.1111/cas.13942. Epub 2019 Feb 15. Cancer Sci. 2019. PMID: 30657619 Free PMC article. Review.
Cited by
- The EphA2 receptor is activated through induction of distinct, ligand-dependent oligomeric structures.
Singh DR, Kanvinde P, King C, Pasquale EB, Hristova K. Singh DR, et al. Commun Biol. 2018 Feb 22;1:15. doi: 10.1038/s42003-018-0017-7. eCollection 2018. Commun Biol. 2018. PMID: 30271902 Free PMC article. - Spatial organization of EphA2 at the cell-cell interface modulates trans-endocytosis of ephrinA1.
Greene AC, Lord SJ, Tian A, Rhodes C, Kai H, Groves JT. Greene AC, et al. Biophys J. 2014 May 20;106(10):2196-205. doi: 10.1016/j.bpj.2014.03.043. Biophys J. 2014. PMID: 24853748 Free PMC article. - Phase separation in immune signalling.
Xiao Q, McAtee CK, Su X. Xiao Q, et al. Nat Rev Immunol. 2022 Mar;22(3):188-199. doi: 10.1038/s41577-021-00572-5. Epub 2021 Jul 6. Nat Rev Immunol. 2022. PMID: 34230650 Free PMC article. Review. - Location, Location, Location: EphB4:Ephrin-B2 Signaling Depends on Its Spatial Arrangement.
Glazier R, Salaita K. Glazier R, et al. Biophys J. 2018 Sep 4;115(5):754-756. doi: 10.1016/j.bpj.2018.07.014. Epub 2018 Jul 23. Biophys J. 2018. PMID: 30082034 Free PMC article. No abstract available. - EphA2 and Src regulate equatorial cell morphogenesis during lens development.
Cheng C, Ansari MM, Cooper JA, Gong X. Cheng C, et al. Development. 2013 Oct;140(20):4237-45. doi: 10.1242/dev.100727. Epub 2013 Sep 11. Development. 2013. PMID: 24026120 Free PMC article.
References
- Lackmann M., Boyd A.W. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci. Signal. 2008;1:re2. - PubMed
- Kinch M.S., Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin. Exp. Metastasis. 2003;20:59–68. - PubMed
- Zelinski D.P., Zantek N.D., Kinch M.S. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous