Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations - PubMed (original) (raw)
Review
Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations
Woody Han et al. Cancer Lett. 2012.
Abstract
The epidermal growth factor receptor (EGFR) pathway is one of the most dysregulated molecular pathways in human cancers. Despite its well-established importance in tumor growth, progression and drug-resistant phenotype over the past several decades, targeted therapy designed to circumvent EGFR has yielded only modest clinical success in cancer patients, except those with non-small cell lung cancer (NSCLC) carrying EGFR activation mutations. However, almost all of these NSCLC patients eventually developed resistance to small molecule EGFR kinase inhibitors. These disappointing outcomes are, in part, due to the high complexity and the interactive nature of the EGFR signaling network. More recent compelling evidence further indicates that EGFR functionality can be dependent on its subcellular location. In this regard, EGFR undergoes translocation into different organelles where it elicits distinctly different functions than its best known activity as a plasma membrane-bound receptor tyrosine kinase. EGFR can be shuttled into the cell nucleus and mitochondrion upon ligand binding, radiation, EGFR-targeted therapy and other stimuli. Nuclear EGFR behaves as transcriptional regulator, tyrosine kinase, and mediator of other physiological processes. The role of mitochondrial EGFR remains poorly understood but it appears to regulate apoptosis and autophagy. While studies using patient tumors have shown nuclear EGFR to be an indicator for poor clinical outcomes in cancer patients, the impact of mitochondrial EGFR on tumor behavior and patient prognosis remains to be defined. Most recently, several lines of evidence suggest that mislocated EGFR may regulate tumor response to therapy and that plasma membrane-bound EGFR elicits survival signals independent of its kinase activity. In light of these recent progresses and discoveries, we will outline in this minireview an emerging line of research that uncovers and functionally characterizes several novel modes of EGFR signaling that take center stage in the cell nucleus, mitochondrion and other subcellular compartments. We will also discuss the clinical implications of these findings in the rationale design for therapeutic strategy that overcomes tumor drug resistance.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures
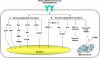
Figure 1. The plasma membrane-bound EGFR/EGFRvIII signaling is consisted of the kinase-dependent and -independent modes of actions
A: Kinase-dependent functions. Upon ligand binding, EGFR becomes activated and phosphorylated at multiple tyrosine residues including those within its kinase domain. Phosphorylated EGFR then recruits and phosphorylates downstream signaling molecules. The major pathways downstream of EGFR include those mediated by PLC-γ–PKC, Ras-Raf-MEK, PI3-K-Akt-mTOR and JAK2-STAT3. In addition, EGFR can directly interact with and phosphorylate STAT3 transcription factor. EGFRvIII is constitutively active independent of ligand stimulation. B: Kinase-independent functions. Co-expression of the kinase-dead EGFR K721M mutant with HER2 rescued the inability of the mutant EGFR to activate Akt and MAPK. Kinase-dead EGFR D813A mutant may activate Akt via undefined mechanisms. Independent of its kinase activity, EGFR also interacts with and stabilizes plasma membrane-bound SGLT1, leading to glucose uptake and increased intracellular glucose levels. Our laboratory recently reported that EGFR and EGFRvIII associated with and sequestered the proapoptotic protein PUMA in the cytoplasm independent on EGF stimulation or its kinase activity. The EGFR-PUMA and EGFRvIII-PUMA interactions contribute to reduced apoptosis and survival.
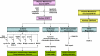
Figure 2. The nuclear mode of EGFR/EGFRvIII signaling network
EGFR nuclear transport can be induced by EGF, Akt phosphorylation, radiation and cisplatin, and conversely, inhibited by lapatinib, dasatinib and celecoxib. Nuclear EGFR has three major functions: (i) gene regulation, (ii) kinase function, and (iii) protein-protein interactions. Via these actions, nuclear EGFR is implicated in a number of physiological and pathological processes, such as proliferation, inflammation, metastasis, DNA repair, and resistance to DNA-damaging radiation and alkylating anti-cancer agents. Nuclear EGFRvIII activates COX-2 gene expression.
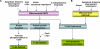
Figure 3. The mitochondrial mode of EGFR/EGFRvIII signaling pathway
A: EGFR mitochondrial import can be constitutive and the extent can be enhanced by apoptosis inducers (staurosporine and anisomycin), EGF, c-Src, Iressa, cetuximab and rapamycin. Conversely, EGFR mitochondrial transport can be blocked by 3-methyadenine (inhibitor of autophagy and PI-3K) and etoposide. Mitochondrial EGFR retains its tyrosine kinase activity and phosphorylates CoxII; however, the consequence of the phosphorylation is not yet defined. The mitochondrial EGFR-CoxII complex can include c-Src; however, the effects of this interaction are still unknown. Furthermore, mitochondrial accumulation of EGFR led to compromised apoptotic response and resistance to Iressa treatments, while the underlying mechanisms are still undetermined. B: EGFRvIII mitochondrial import is constitutive and can be further enhanced by apoptosis inducers (staurosporine and anisomycin) and by Iressa. Mitochondrial accumulation of EGFRvIII rendered tumor cells highly resistant to apoptotic death and to Iressa treatments, while the mechanisms are still not identified.
Similar articles
- Nuclear mode of the EGFR signaling network: biology, prognostic value, and therapeutic implications.
Lo HW. Lo HW. Discov Med. 2010 Jul;10(50):44-51. Discov Med. 2010. PMID: 20670598 Free PMC article. Review. - The nuclear epidermal growth factor receptor signaling network and its role in cancer.
Brand TM, Iida M, Li C, Wheeler DL. Brand TM, et al. Discov Med. 2011 Nov;12(66):419-32. Discov Med. 2011. PMID: 22127113 Free PMC article. Review. - Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC).
Hsu PC, Jablons DM, Yang CT, You L. Hsu PC, et al. Int J Mol Sci. 2019 Aug 5;20(15):3821. doi: 10.3390/ijms20153821. Int J Mol Sci. 2019. PMID: 31387256 Free PMC article. Review. - Targeting the epidermal growth factor receptor in non-small cell lung cancer cells: the effect of combining RNA interference with tyrosine kinase inhibitors or cetuximab.
Chen G, Kronenberger P, Teugels E, Umelo IA, De Grève J. Chen G, et al. BMC Med. 2012 Mar 21;10:28. doi: 10.1186/1741-7015-10-28. BMC Med. 2012. PMID: 22436374 Free PMC article. - HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors.
Gusenbauer S, Vlaicu P, Ullrich A. Gusenbauer S, et al. Oncogene. 2013 Aug 15;32(33):3846-56. doi: 10.1038/onc.2012.396. Epub 2012 Oct 8. Oncogene. 2013. PMID: 23045285
Cited by
- Potential crosstalk between cofilin-1 and EGFR pathways in cisplatin resistance of non-small-cell lung cancer.
Müller CB, De Bastiani MA, Becker M, França FS, Branco MA, Castro MA, Klamt F. Müller CB, et al. Oncotarget. 2015 Feb 28;6(6):3531-9. doi: 10.18632/oncotarget.3471. Oncotarget. 2015. PMID: 25784483 Free PMC article. - Association of epidermal growth factor and epidermal growth factor receptor polymorphisms with the risk of hepatitis B virus-related hepatocellular carcinoma in the population of North China.
Wu J, Zhang W, Xu A, Zhang L, Yan T, Li Z, Wu X, Zhu X, Ma J, Li K, Li H, Liu Y. Wu J, et al. Genet Test Mol Biomarkers. 2013 Aug;17(8):595-600. doi: 10.1089/gtmb.2013.0031. Epub 2013 Jun 22. Genet Test Mol Biomarkers. 2013. PMID: 23790025 Free PMC article. Clinical Trial. - The Correlation between EGFR Mutation Status and DNA Content of Lung Adenocarcinoma Cells in Pleural Effusion.
Du Y, Guo X, Wang R, Ma Y, Zhang Y, Liu Y, Dong L, Wu J, Ji X, Wang H. Du Y, et al. J Cancer. 2020 Feb 6;11(8):2265-2272. doi: 10.7150/jca.38615. eCollection 2020. J Cancer. 2020. PMID: 32127953 Free PMC article. - Exposure of Barrett's and esophageal adenocarcinoma cells to bile acids activates EGFR-STAT3 signaling axis via induction of APE1.
Bhat AA, Lu H, Soutto M, Capobianco A, Rai P, Zaika A, El-Rifai W. Bhat AA, et al. Oncogene. 2018 Nov;37(46):6011-6024. doi: 10.1038/s41388-018-0388-8. Epub 2018 Jul 10. Oncogene. 2018. PMID: 29991802 Free PMC article. - Modulating the structure of EGFR with UV light: new possibilities in cancer therapy.
Correia M, Thiagarajan V, Coutinho I, Gajula GP, Petersen SB, Neves-Petersen MT. Correia M, et al. PLoS One. 2014 Nov 11;9(11):e111617. doi: 10.1371/journal.pone.0111617. eCollection 2014. PLoS One. 2014. PMID: 25386651 Free PMC article.
References
- Cohen S, Carpenter G, King L., Jr. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255:4834–4842. - PubMed
- Cohen S, Ushiro H, Stoscheck C, Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982;257:1523–1531. - PubMed
- Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. - PubMed
- Cohen S, Carpenter G, King L., Jr. Epidermal growth factor-receptor-protein kinase interactions. Prog Clin Biol Res. 1981;66(Pt A):557–567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous