The zebra finch paradox: song is little changed, but number of neurons doubles - PubMed (original) (raw)
The zebra finch paradox: song is little changed, but number of neurons doubles
Clare Walton et al. J Neurosci. 2012.
Abstract
New neurons are added to the high vocal center (HVC) of adult males in seasonally breeding songbirds such as the canary (Serinus canaria) that learns new songs in adulthood, and the song sparrow (Melospiza melodia) that does not. In both cases, the new neurons numerically replace others that have died, resulting in a seasonal fluctuation in HVC volume and neuron number. Peaks in neuronal replacement in both species occur in the fall when breeding is over and song is variable. New neurons are added, too, to the HVC of zebra finches (Taeniopygia guttata) that do not learn new songs in adulthood and whose song remains stereotyped throughout the year. Here, we show that, in contrast to the observations in seasonal songbirds, neurons added to the zebra finch HVC are not part of a replacement process. Rather, they lead to a doubling in the number of neurons that project from HVC to the robust nucleus of the arcopallium (RA). As this happens, HVC volume remains constant and the packing density of its neurons increases. The HVC-RA neurons are part of a descending pathway that carries the pattern of learned song; some HVC-RA neurons are also responsive to song playback. The addition of HVC-RA neurons happens in zebra finches housed singly, but becomes more acute if the birds are housed communally. We speculate that new neurons added to the adult HVC may help with the production or perception of learned song, or both.
Figures
Figure 1.
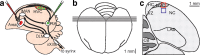
Diagrams illustrating the surgery and brain areas that were sampled for neuron counts in HVC and NC in Experiment 1. a, Schematic illustration of the double labeling surgery. Green fluorescent cholera toxin B (Alexa-488-CTB, Invitrogen) is injected bilaterally into nucleus RA and red fluorescent cholera toxin B (Alexa-555-CTB, Invitrogen) is injected bilaterally into Area X. Birds are killed after 6 d to allow retrograde transport of the tracers back to the cell bodies in HVC. b, Dorsal view of adult zebra finch brain showing the placement and orientation of sections for neuron counts in HVC and NC. c, Frontal diagram in the plane of section shown in (b) representing part of one of the middle sections through HVC. The boxes show an example of the area included in a confocal Z-stack and used for neuron counts in HVC (red) and NC (blue). nXIIts, The tracheosyringeal portion of the nucleus of the twelfth cranial nerve; DLM, medial portion of the dorsolateral thalamus; HC, hippocampus; LAD, dorsal archopallial lamina.
Figure 2.
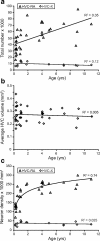
The total number and packing density of HVC-RA neurons increases between 90 d and 11 years of age in the adult male zebra finch whereas the number and density of HVC-X neurons do not change over the same age range. a, There is a significant increase in the total number of HVC-RA projection neurons between 90 d and 11 years of age (p < 0.0005), but there is no change in the number of HVC-X projection neurons over the same age range (p = 0.19). b, HVC volume does not change with age between 90 d and 11 years (p = 0.67). c, The packing density of HVC-RA neurons in HVC significantly increases between 90 d and 11 years of age in the adult male zebra finch and this relationship is best modeled using logarithmic regression (p = 1.8 × 10−10). The density of HVC-X neurons does not change with age over the same age range (p = 0.56). For all three graphs, each data point represents the average of the left and right HVC from one bird.
Figure 3.
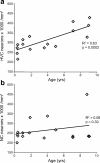
The packing density of Hu-positive neurons significantly increases with age in the HVC, but not in an area of NC ventral to HVC, in adult male zebra finches. a, The packing density of Hu-positive neurons in HVC significantly increases between 90 d and 9 years of age in adult male zebra finches (p = 0.0002). b, In the same group of birds, there is no significant change in the packing density of Hu-positive neurons with age in an area of NC just ventral to HVC (see Fig. 1_c_). Each data point represents the counts from either left or right HVC within one bird. There was no systematic difference in neuron density between the left and right hemispheres in either HVC or NC densities.
Figure 4.
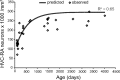
A model that assumes complete survival of new HVC-RA neurons approximates the age-related changes we see in the HVC-RA neuron density with age. Using a rate equation derived from data in Wang et al. (2002) showing how HVC neuron recruitment changes with age in adult zebra finches, a set of predicted values for the total number of HVC-RA neurons that would be present in birds of different ages was generated. These predicted values assumed that every neuron that was recruited to HVC and survived for 4 months in Wang et al. (2002) would go on to survive indefinitely, and that neuron addition was accompanied by no loss of the preexisting HVC-neuron cohort. Predicted values for total HVC-RA neuron number were converted to HVC-RA neuron density using a canonical volume for HVC. The predicted values (gray line) that assume complete survival of new HVC-RA neurons were compared with the observed values for HVC-RA density and age (diamonds). The model comes close to the real change in HVC-RA neuron density with age that we have measured (_R_2 = 0.65).
Figure 5.
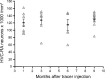
The number of HVC-RA neurons retrogradely labeled at 95 d of age does not change during the following 9 months. Counts of HVC-RA neurons retrogradely labeled with latex microspheres injected into RA at 95 d of age are similar after 1, 3, 6, and 9 months survival time. Each data point represents the mean between left and right HVC within one bird. Group means are represented as black bars. Error bars represent SEM.
Figure 6.
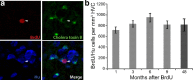
Neurons born at 92–94 d of age are still present in HVC 4 years later. a, Many BrdU/Hu double-positive neurons were present in HVC of birds killed 4 years after BrdU injections at 92–94 d of age. A significant number of them could also be retrogradely labeled by green cholera toxin B from an injection into RA 6 d before death. Arrowheads point to a BrdU/Hu/CTB triple-labeled neuron. b, Counts of BrdU/Hu double-positive neurons in HVC at various survival times after BrdU injections at 92–94 d of age revealed that there was no loss of labeled cells between 1 month and 4 years. Error bars represent SEM.
Figure 7.
Microspheres can be transported from RA after the initial injection but the number of additionally labeled neurons does not dramatically alter the final HVC-RA neuron counts. a, At all time points after microsphere injection, some of the BrdU-positive neurons were also labeled with microspheres from RA, even though they would not have been connected to RA at the time of microsphere injection. The percentage of BrdU-positive neurons labeled with microspheres was not significantly different between survival times (p = 0.14). Across all survival times, on average, 7.5% of the BrdU-positive neurons were retrogradely labeled with microspheres. Each data point represents the mean between left and right HVC of one bird. Group means are represented as black bars. Error bars represent SEM. b, The total number of microsphere-positive HVC-RA neurons present at each survival time (black diamonds) was corrected for potential extra labeled neurons that were recruited after the microsphere injection but still took up microspheres from a deposit in RA. Extra labeled neurons were estimated for each survival time using published data on the rate of neuronal recruitment to HVC (Wang et al., 2002) and a mean probability that 7.5% of them will take up microspheres. Even correcting for these potential extra labeled neurons (white diamonds), there is no significant difference between the total number of microsphere-positive HVC-RA neurons present at 1, 3, 6, or 9 months after microsphere injection at 95 d of age (p = 0.57).
Figure 8.
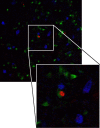
The number of BrdU-positive ventricular zone cells declines with survival time after BrdU injection. a, BrdU-positive ventricular zone cells were identified by a green BrdU-positive nucleus embedded in the ventricular surface above HVC (shown with arrowheads). Three positive cells, all negative for the blue Hu neuron stain, can be seen in this example. Red spots represent microspheres retrogradely transported from an injection into RA. b, There is a nonlinear decrease in the number of BrdU-positive ventricular zone cells as the survival time after BrdU injection increases, which most likely represents continuing division of the BrdU-positive progenitors after the time of initial labeling. Each data point represents the average of left and right ventricular zone counts for each bird. Group means are shown as black bars. The trend line represents the regression line through the group means and has the equation y = 190_x_−0.5 (_R_2 = 0.99).
Figure 9.
Four-year-old adult-born neurons in HVC do not express interneuron markers. A mixture of antibodies against the calcium-binding proteins calbindin, calretinin, and parvalbumin stains many interneurons in HVC (blue) but there is no colocalization between the interneuron markers and BrdU (red) in birds that had received BrdU injections at 90 d of age and were killed 4 years later. HVC-RA neurons are labeled with CTB retrograde tracer injected into RA before killing (green). The enlarged panel shows the BrdU-positive nucleus of a retrogradely labeled HVC-RA neuron surrounded by three interneurons positive for the mix of calcium binding proteins.
Figure 10.
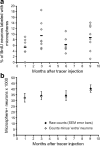
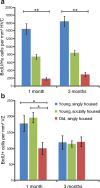
Age and social environment affect the recruitment of new neurons to adult HVC but not the survival of new neurons between 1 and 3 months. a, One month after BrdU injection, the young, socially housed adults had recruited twice as many BrdU-positive neurons to HVC and the old, singly housed birds had recruited four times fewer BrdU-positive neurons to HVC than the young, singly housed birds. There was no loss of BrdU-positive neurons in HVC between 1 and 3 month survival in any of the three groups. b, There was no difference in the number of BrdU-positive VZ cells between the young, socially housed and the young, singly housed groups at either 1 or 3 months survival times, and both young groups showed a significant reduction in the number of BrdU-positive neurons between 1 and 3 months. Old, singly housed birds had half the number of BrdU-positive VZ cells 1 months after BrdU injections than the number in either young group but they did not show any loss in the number of labeled VZ cells between 1 and 3 months, suggesting no further division of the progenitors occurred. *p < 0.01; **p < 0.0001.
Figure 11.
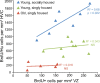
The number of new neurons in HVC is only correlated with the number of dividing progenitors in the young, socially housed birds. BrdU-positive neurons in HVC were significantly correlated with the number of BrdU-positive cells in the ventricular zone in the young, aviary-housed birds (_R_2 = 0.68, p = 0.022) but not in the young, singly housed or old, singly housed groups.
Similar articles
- DARPP-32 distinguishes a subset of adult-born neurons in zebra finch HVC.
Aronowitz JV, Kirn JR, Pytte CL, Aaron GB. Aronowitz JV, et al. J Comp Neurol. 2022 Apr;530(5):792-803. doi: 10.1002/cne.25245. Epub 2021 Nov 15. J Comp Neurol. 2022. PMID: 34545948 Free PMC article. - Is neurogenesis in two songbird species related to their song sequence variability?
Polomova J, Lukacova K, Bilcik B, Kubikova L. Polomova J, et al. Proc Biol Sci. 2019 Jan 30;286(1895):20182872. doi: 10.1098/rspb.2018.2872. Proc Biol Sci. 2019. PMID: 30963944 Free PMC article. - Adult Neurogenesis Leads to the Functional Reconstruction of a Telencephalic Neural Circuit.
Cohen RE, Macedo-Lima M, Miller KE, Brenowitz EA. Cohen RE, et al. J Neurosci. 2016 Aug 24;36(34):8947-56. doi: 10.1523/JNEUROSCI.0553-16.2016. J Neurosci. 2016. PMID: 27559175 Free PMC article. - Birth, migration, incorporation, and death of vocal control neurons in adult songbirds.
Alvarez-Buylla A, Kirn JR. Alvarez-Buylla A, et al. J Neurobiol. 1997 Nov;33(5):585-601. J Neurobiol. 1997. PMID: 9369461 Review. - A species-specific view of song representation in a sensorimotor nucleus.
Alliende J, Lehongre K, Del Negro C. Alliende J, et al. J Physiol Paris. 2013 Jun;107(3):193-202. doi: 10.1016/j.jphysparis.2012.08.004. Epub 2012 Aug 30. J Physiol Paris. 2013. PMID: 22960663 Review.
Cited by
- The impact of spike timing precision and spike emission reliability on decoding accuracy.
Nicola W, Newton TR, Clopath C. Nicola W, et al. Sci Rep. 2024 May 8;14(1):10536. doi: 10.1038/s41598-024-58524-7. Sci Rep. 2024. PMID: 38719897 Free PMC article. - Song system neuroanatomy, and immediate early gene expression in a finch species with extensive male and female song.
Rose EM, Haakenson CM, Patel A, Gaind S, Shank BD, Ball GF. Rose EM, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Sep;210(5):735-749. doi: 10.1007/s00359-023-01651-9. Epub 2023 Jul 12. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37436439 - A juvenile locomotor program promotes vocal learning in zebra finches.
Liu WC, Landstrom M, Cealie M, MacKillop I. Liu WC, et al. Commun Biol. 2022 Jun 10;5(1):573. doi: 10.1038/s42003-022-03533-3. Commun Biol. 2022. PMID: 35689094 Free PMC article. - DARPP-32 distinguishes a subset of adult-born neurons in zebra finch HVC.
Aronowitz JV, Kirn JR, Pytte CL, Aaron GB. Aronowitz JV, et al. J Comp Neurol. 2022 Apr;530(5):792-803. doi: 10.1002/cne.25245. Epub 2021 Nov 15. J Comp Neurol. 2022. PMID: 34545948 Free PMC article. - Unilateral vocal nerve resection alters neurogenesis in the avian song system in a region-specific manner.
Aronowitz JV, Perez A, O'Brien C, Aziz S, Rodriguez E, Wasner K, Ribeiro S, Green D, Faruk F, Pytte CL. Aronowitz JV, et al. PLoS One. 2021 Aug 31;16(8):e0256709. doi: 10.1371/journal.pone.0256709. eCollection 2021. PLoS One. 2021. PMID: 34464400 Free PMC article.
References
- Adar E, Lotem A, Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res. 2008;187:178–184. - PubMed
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials