Transcription factor RORα is critical for nuocyte development - PubMed (original) (raw)
. 2012 Jan 22;13(3):229-36.
doi: 10.1038/ni.2208.
Jennifer A Walker, Helen E Jolin, Lesley F Drynan, Emily Hams, Ana Camelo, Jillian L Barlow, Daniel R Neill, Veera Panova, Ute Koch, Freddy Radtke, Clare S Hardman, You Yi Hwang, Padraic G Fallon, Andrew N J McKenzie
Affiliations
- PMID: 22267218
- PMCID: PMC3343633
- DOI: 10.1038/ni.2208
Transcription factor RORα is critical for nuocyte development
See Heng Wong et al. Nat Immunol. 2012.
Abstract
Nuocytes are essential in innate type 2 immunity and contribute to the exacerbation of asthma responses. Here we found that nuocytes arose in the bone marrow and differentiated from common lymphoid progenitors, which indicates they are distinct, previously unknown members of the lymphoid lineage. Nuocytes required interleukin 7 (IL-7), IL-33 and Notch signaling for development in vitro. Pro-T cell progenitors at double-negative stage 1 (DN1) and DN2 maintained nuocyte potential in vitro, although the thymus was not essential for nuocyte development. Notably, the transcription factor RORα was critical for the development of nuocytes and their role in the expulsion of parasitic worms.
Figures
Figure 1
Nuocytes are distinct from CLPs. (a) Flow cytometry profiles for nuocyte (Lin−ICOS+T1/ST2+ Flt3−) and CLP (Lin−IL-7Rα+Flt3+) in naïve Lin−IL-7Rα+ cells. Representative of three repeat experiments. (b) ELISA for IL-4, IL-5 and IL-13 in supernatants from purified Lin−IL-7Rα+Flt3− and Lin−IL-7Rα+Flt3+ cells cultured at 1 × 105/ml for 72 hrs. Representative of two experiments with duplicate wells in each. (c, d) Nuocyte numbers from bone marrow (c) and peritoneum (d) in wild-type (WT) and _Il7ra_−/− mice treated with PBS or IL-25 intraperitoneally. * = p < 0.05; ** = p < 0.01. Representative data from two independent experiments with 4 to 5 mice per group.
Figure 2
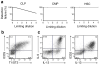
Notch signalling is essential for the in vitro generation of nuocytes from CLPs. (a) Flow cytometry profiles of CLPs cultured on OP9 or OP9-DL1 stromal cells and analysed for the expression of T (CD3+), B (CD19+) and nuocyte (ICOS+T1/ST2+) markers on day 20. (b) ELISA of culture supernatants collected from the above OP9-DL1 cultures on the days indicated. Data are representative of two independent experiments.
Figure 3
Progenitor frequencies support nuocyte lymphoid origin. (a) Clonal expansion from single or 5 CLPs, CMPs or HSCs (means of 60 - 84 wells of each) sorted onto OP9-DL1 stromal cells and cultured in the presence of IL-7 and IL-33 for 18 days. (b) Flow cytometry analysis of ICOS and T1/ST2, and (c) intracellular cytokine expression by a representative CLP-derived clone at day 18.
Figure 4
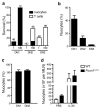
Adoptive transfer of CLPs gives rise to nuocytes. (a) Flow cytometric detection of nuocytes (B220−CD3−ICOS+) from donor HSCs or CLPs (CD45.1) in mesenteric lymph node following IL-25 treated mice ~6 weeks after reconstitution. (b) Flow cytometry of peritoneal cells for the presence of donor HSCs or CLPs CD45.1 cells within the eosinophil (SSChiSiglecF+), B/T cells (B220+/CD3+) and nuocyte (Lin−ICOS+) populations. Data are representative of three independent experiments with 5 to 6 mice per group.
Figure 5
DN1 and DN2 thymocytes retain the plasticity to differentiate into nuocytes. Thymocyte subsets were cultured on OP9-DL1 stromal cells at 1 or 100 cells per well in the presence of IL-7 and IL-33. (a) Clonal survival after 2 to 5 weeks. The numbers above the bars indicate the numbers of starting wells. ND, not detected. (b,c) Flow cytometry detection of nuocytes (Lin−ICOS+T1/ST2+) in IL-7 + IL-33 cultures after 24 days (b) and 38 days (c). (d) Flow cytometry analysis of nuocytes (Lin−ICOS+T1/ST2+) in nude mice following IL-25 administration for 3 days.
Figure 6
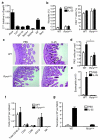
Rorα is required for nuocyte development. (a) qPCR analysis of gene expression in ex vivo nuocytes. (b) Flow cytometric analysis of nuocytes and CD4+ T cells in _Rora_sg/sg and wildtype mice in mesenteric lymph node (MLN) after 4 daily doses of IL-25, or PBS. Combined data from two repeat experiments with 3 - 6 mice per group. (c) PAS stained histology sections of intestine following IL-25 treatment. (d) Enumeration of PAS+ (goblet) cells in intestine. (e) Flow cytometry analysis of eosinophils in peritoneal lavage after IL-25 treatment. (f) Flow cytometry analysis of splenocytes following IL-25 treatment ~six weeks after total bone marrow reconstitution. (g) Flow cytometry analysis of nuocyte (Lin−ICOS+T1/ST2+) development from _Rora_sg/sg bone marrow transfer. * = p < 0.05. Data are representative of two independent experiments with 2 - 4 mice per group.
Figure 7
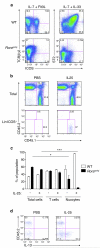
Intrinsic Rorα is required for nuocyte development. (a) Flow cytometry analysis of T cells and nuocytes derived from _Rora_sg/sg or wildtype CLPs cultured in vitro for 16 days. Data are representative of two independent experiments. (b) Flow cytometry analysis of total cells (upper panel) and nuocytes (lower panel) in the CD45.1 and CD45.2 cell compartments of MLN following IL-25 treatment ~six weeks after mixed chimera bone marrow transfers. (c) Flow cytometry analysis of the CD45.1 and CD45.2 reconstituting cells following PBS or IL-25 treatment. (d) Intracellular IL-13 expression in the Lin−CD45.1 and Lin−CD45.2 cell compartments following PBS or IL-25 treatment ~six weeks after mixed chimera bone marrow transfers. * = p < 0.05; *** = p < 0.0001. Data are representative of two independent experiments with 3 - 5 mice per group.
Figure 8
_Rora_sg/sg mice fail to expel N. brasiliensis efficiently. (a) Worm burdens at day 6 post-infection. Data are combined from two independent experiments. WT n = 11, _Rora_sg/sg n = 4. (b) Flow cytometry analysis of nuocytes (Lin−ICOS+T1/ST2+) in MLN at day 6 post-infection. (c) Worm burdens at day 5 post-infection in irradiated WT recipients reconstituted with either WT or _Rora_sg/sg total bone marrow six weeks previously. Flow cytometry analysis of (d) nuocytes (Lin−ICOS+T1/ST2+) and (e) CD4+ T cells, in MLN at day 5 post-infection in irradiated WT recipients reconstituted with either WT or _Rora_sg/sg total bone marrow six weeks previously. NS = non-significant; * = p < 0.05; ** = p < 0.01. Data are representative of two independent experiments with 4 - 5 mice per group
Similar articles
- Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity.
Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Neill DR, et al. Nature. 2010 Apr 29;464(7293):1367-70. doi: 10.1038/nature08900. Epub 2010 Mar 3. Nature. 2010. PMID: 20200518 Free PMC article. - RORα is a critical checkpoint for T cell and ILC2 commitment in the embryonic thymus.
Ferreira ACF, Szeto ACH, Heycock MWD, Clark PA, Walker JA, Crisp A, Barlow JL, Kitching S, Lim A, Gogoi M, Berks R, Daly M, Jolin HE, McKenzie ANJ. Ferreira ACF, et al. Nat Immunol. 2021 Feb;22(2):166-178. doi: 10.1038/s41590-020-00833-w. Epub 2021 Jan 11. Nat Immunol. 2021. PMID: 33432227 Free PMC article. - The transcription factor RORα is required for the development of type 1 innate lymphoid cells in adult bone marrow.
Abe S, Kagao M, Asahi T, Kato R, Tani-Ichi S, Shimba A, Ishibashi R, Miyachi H, Kitano S, Miyazaki M, Rodewald HR, Toyoshima F, Ikuta K. Abe S, et al. J Immunol. 2025 Apr 1;214(4):575-581. doi: 10.1093/jimmun/vkaf001. J Immunol. 2025. PMID: 40079843 - Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development.
Hong C, Luckey MA, Park JH. Hong C, et al. Semin Immunol. 2012 Jun;24(3):151-8. doi: 10.1016/j.smim.2012.02.002. Epub 2012 Mar 14. Semin Immunol. 2012. PMID: 22421571 Free PMC article. Review. - Type 2 innate lymphoid cells: new players in asthma and allergy.
Scanlon ST, McKenzie AN. Scanlon ST, et al. Curr Opin Immunol. 2012 Dec;24(6):707-12. doi: 10.1016/j.coi.2012.08.009. Epub 2012 Sep 15. Curr Opin Immunol. 2012. PMID: 22985480 Review.
Cited by
- Rhinovirus C Infection Induces Type 2 Innate Lymphoid Cell Expansion and Eosinophilic Airway Inflammation.
Rajput C, Han M, Ishikawa T, Lei J, Goldsmith AM, Jazaeri S, Stroupe CC, Bentley JK, Hershenson MB. Rajput C, et al. Front Immunol. 2021 Apr 22;12:649520. doi: 10.3389/fimmu.2021.649520. eCollection 2021. Front Immunol. 2021. PMID: 33968043 Free PMC article. - The origin and role of innate lymphoid cells in the lung.
Lai DM, Shu Q, Fan J. Lai DM, et al. Mil Med Res. 2016 Aug 19;3:25. doi: 10.1186/s40779-016-0093-2. eCollection 2016. Mil Med Res. 2016. PMID: 27547445 Free PMC article. Review. - RORα, a potential tumor suppressor and therapeutic target of breast cancer.
Du J, Xu R. Du J, et al. Int J Mol Sci. 2012 Nov 26;13(12):15755-66. doi: 10.3390/ijms131215755. Int J Mol Sci. 2012. PMID: 23443091 Free PMC article. - The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development.
Yu Y, Wang C, Clare S, Wang J, Lee SC, Brandt C, Burke S, Lu L, He D, Jenkins NA, Copeland NG, Dougan G, Liu P. Yu Y, et al. J Exp Med. 2015 Jun 1;212(6):865-74. doi: 10.1084/jem.20142318. Epub 2015 May 11. J Exp Med. 2015. PMID: 25964371 Free PMC article. - Role of innate lymphocytes in infection and inflammation.
Koyasu S, Moro K. Koyasu S, et al. Front Immunol. 2012 May 7;3:101. doi: 10.3389/fimmu.2012.00101. eCollection 2012. Front Immunol. 2012. PMID: 22783250 Free PMC article.
References
- Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. - PubMed
- Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. - PubMed
- Neill DR, McKenzie AN. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011;27:214–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases