Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica - PubMed (original) (raw)
Comparative Study
Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica
Lukmanee Tradtrantip et al. Ann Neurol. 2012 Mar.
Abstract
Objective: Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system. Circulating autoantibodies (NMO-immunoglobulin [Ig]G) against astrocyte water channel aquaporin-4 (AQP4) cause complement- and cell-mediated astrocyte damage with consequent neuroinflammation and demyelination. Current NMO therapies, which have limited efficacy, include immunosuppression and plasma exchange. The objective of this study was to develop a potential new NMO therapy based on blocking of pathogenic NMO-IgG binding to its target, AQP4.
Methods: We generated nonpathogenic recombinant monoclonal anti-AQP4 antibodies that selectively block NMO-IgG binding to AQP4. These antibodies comprise a tight-binding anti-AQP4 Fab and a mutated Fc that lacks functionality for complement- and cell-mediated cytotoxicity. The efficacy of the blocking antibodies was studied using cell culture, spinal cord slice, and in vivo mouse models of NMO.
Results: In AQP4-expressing cell cultures, the nonpathogenic competing antibodies blocked binding of NMO-IgG in human sera, reducing to near zero complement- and cell-mediated cytotoxicity. The antibodies prevented the development of NMO lesions in an ex vivo spinal cord slice model of NMO and in an in vivo mouse model, without causing cytotoxicity.
Interpretation: Our results provide proof of concept for a therapy of NMO with blocking antibodies. The broad efficacy of antibody inhibition is likely due to steric competition because of its large physical size compared to AQP4. Blocker therapy to prevent binding of pathogenic autoantibodies to their targets may be useful for treatment of other autoimmune diseases as well.
Copyright © 2011 American Neurological Association.
Figures
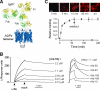
Figure 1. High-affinity monoclonal, recombinant anti-AQP4 antibody for antibody blocking therapy
a. Crystal structure of AQP4 tetramer shown on the same scale with that of an IgG1 antibody. b. Surface plasmon resonance measurement of recombinant antibody binding to AQP4-reconstituted proteoliposomes showing binding / unbinding kinetics of rAb-53 (left) at different concentrations, and different NMO rAbs (right) at fixed concentration. c. Binding and unbinding kinetics rAb-53 (25 μg/ml) to AQP4-expressing U87MG cells. Binding measured by incubation with rAb-53 for specified times followed by rinsing, fixation and fluorescent secondary antibody addition. Washout measured after 60 min incubation with rAb-53 followed by washout with antibody-free buffer for specified times. Top: Representative micrographs showing cell surface staining by rAb-53 (red). Bottom: Averaged binding data (mean ± S.E., n=4).
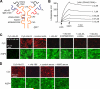
Figure 2. Mutated, non-pathogenic rAb-53 monoclonal antibodies blocks binding of pathogenic NMO-IgG to AQP4
a. Schematic of rAb-53 showing heavy (VH) and light (VL) chain variable regions, light chain constant region (CL), and IgG1 heavy chain constant regions (CH1-CH3). Locations of amino acid mutations introduced in the CH2 domain to reduce CDC (K322A), ADCC (K326W/E333S) or both (L234A/L235A). b. Surface plasmon resonance measurements of binding and washout of a mutated rAb-53 (L234A/L235A) to AQP4-reconstituted proteoliposomes. c. Mutated rAb-53 blocks binding of Cy3-labeled (non-mutated) rAb-53 to AQP4-expressing cells. Cy3 fluorescence imaged in AQP4-null (left-most panel) or AQP4-expressing (other panels) cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of indicated (unlabeled) antibodies at 100 μg/ml. d. Unrelated monoclonal NMO antibodies and human NMO serum block AQP4 binding of Cy3-labeled rAb-53. Cy3 fluorescence imaged in cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of 10% control (non-NMO) or NMO patient serum, or 100 μg/ml recombinant NMO monoclonal antibody rAb-186.
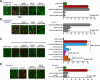
Figure 3. Mutated non-pathogenic rAb-53 prevents CDC and ADCC in NMO-IgG-exposed AQP4-expressing cells
a. Live/dead cell assay after 90 min exposure of AQP4-expressing CHO cells to human complement together with control (non-NMO) mAb or rAb-53 (2.5 μg/ml, non-mutated or mutated). Percentage dead cells summarized at the right (mean ± S.E., n=4−6, * P < 0.001 compared to rAb-53 alone). b. Assay as in A done with complement + rAb-53, in the presence of 12.5 μg/ml of the indicated blocking antibodies. c. Live/dead cell assay after 60 min exposure to control (non-NMO) serum or NMO patient sera in the presence of complement, and the absence or presence of indicated blocking antibodies. d. ADCC assay done using AQP4-expressing CHO cells incubated with NK-cells together with control (non-NMO) mAb or rAb-53 or blocking antibodies (individually), or rAb-53 and blocking antibodies together.
Figure 4. Blocking antibody reduces NMO-like lesions in mouse brain in vivo produced by intracerebral injection of NMO-IgG and human complement
a. Panel of mouse brain sections at 24 h after intracerebral injection, stained with hematoxylin and eosin (H&E) and Luxol fast blue (myelin), and immunostained brown for AQP4 (AQP4) and C5b-9 (activated complement). Intracerebral injections were made of NMO-IgG (purified IgG from NMO serum) and human complement, without or with blocking antibody (rAbmut), with controls (control IgG, AQP4 knockout mice, rAbmut alone). Pink line indicates areas of absent Luxol fast blue staining or AQP4 immunoreactivity. Black line outlines the injected hemisphere and shows needle tract. Arrows, neutrophils; arrowheads, perivascular C5b-9 immunoreactivity; V, vessel. Bar, 50 μm. b. AQP4 and myelin loss quantified as % area outlined with pink / area outlined with black (S.E.M., 5 mice per group, * P < 0.01). c. % myelin and AQP4 loss shown for five pairs of mice, each pair injected with NMO-IgG from a different NMO patient with human complement, without or with rAbmut.
Figure 5. Blocking antibody reduces NMO-like lesions produced by NMO-IgG and human complement in ex vivo spinal cord slice cultures
a. Ex vivo spinal cord slice culture model in which slices were cultured for 7 days, followed by 3 days in the presence of NMO-IgG (purified IgG from NMO serum) and human complement, without or with blocking antibody (rAbmut). Immunostaining shown for AQP4, GFAP and myelin. Controls include non-NMO IgG, NMO-IgG or rAbmut alone, rAbmut with complement, and slice cultures from AQP4 null mice. b. NMO lesion scores (see Methods) (S.E.M., n=4−5, * P < 0.001).
Comment in
- White matter disease: A novel approach to treatment of neuromyelitis optica.
Wood H. Wood H. Nat Rev Neurol. 2011 Nov 22;7(12):656. doi: 10.1038/nrneurol.2011.188. Nat Rev Neurol. 2011. PMID: 22105212 No abstract available. - Re-engineering of pathogenic aquaporin 4-specific antibodies as molecular decoys to treat neuromyelitis optica.
Steinman L, Zamvil SS. Steinman L, et al. Ann Neurol. 2012 Mar;71(3):287-8. doi: 10.1002/ana.23538. Ann Neurol. 2012. PMID: 22451198 No abstract available.
Similar articles
- Isotetrandrine Reduces Astrocyte Cytotoxicity in Neuromyelitis Optica by Blocking the Binding of NMO-IgG to Aquaporin 4.
Sun M, Wang J, Zhou Y, Wang Z, Jiang Y, Li M. Sun M, et al. Neuroimmunomodulation. 2016;23(2):98-108. doi: 10.1159/000444530. Epub 2016 Apr 12. Neuroimmunomodulation. 2016. PMID: 27064690 - Affinity-matured 'aquaporumab' anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders.
Duan T, Tradtrantip L, Phuan PW, Bennett JL, Verkman AS. Duan T, et al. Neuropharmacology. 2020 Jan 1;162:107827. doi: 10.1016/j.neuropharm.2019.107827. Epub 2019 Oct 22. Neuropharmacology. 2020. PMID: 31654702 Free PMC article. - Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly.
Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Rossi A, et al. Glia. 2012 Dec;60(12):2027-39. doi: 10.1002/glia.22417. Epub 2012 Sep 14. Glia. 2012. PMID: 22987455 Free PMC article. - Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies.
Ratelade J, Verkman AS. Ratelade J, et al. Int J Biochem Cell Biol. 2012 Sep;44(9):1519-30. doi: 10.1016/j.biocel.2012.06.013. Epub 2012 Jun 17. Int J Biochem Cell Biol. 2012. PMID: 22713791 Free PMC article. Review. - Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.
Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Verkman AS, et al. Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
- Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later.
Pittock SJ, Lucchinetti CF. Pittock SJ, et al. Ann N Y Acad Sci. 2016 Feb;1366(1):20-39. doi: 10.1111/nyas.12794. Epub 2015 Jun 10. Ann N Y Acad Sci. 2016. PMID: 26096370 Free PMC article. Review. - Challenges and opportunities in designing clinical trials for neuromyelitis optica.
Weinshenker BG, Barron G, Behne JM, Bennett JL, Chin PS, Cree BA, de Seze J, Flor A, Fujihara K, Greenberg B, Higashi S, Holt W, Khan O, Knappertz V, Levy M, Melia AT, Palace J, Smith TJ, Sormani MP, Van Herle K, VanMeter S, Villoslada P, Walton MK, Wasiewski W, Wingerchuk DM, Yeaman MR; Guthy-Jackson Charitable Foundation International Clinical Consortium. Weinshenker BG, et al. Neurology. 2015 Apr 28;84(17):1805-15. doi: 10.1212/WNL.0000000000001520. Epub 2015 Apr 3. Neurology. 2015. PMID: 25841026 Free PMC article. Review. - Eosinophil pathogenicity mechanisms and therapeutics in neuromyelitis optica.
Zhang H, Verkman AS. Zhang H, et al. J Clin Invest. 2013 May;123(5):2306-16. doi: 10.1172/JCI67554. Epub 2013 Apr 8. J Clin Invest. 2013. PMID: 23563310 Free PMC article. - Treatment strategies for neuromyelitis optica.
Huang TL, Lin KH, Wang JK, Tsai RK. Huang TL, et al. Tzu Chi Med J. 2018 Oct-Dec;30(4):204-208. doi: 10.4103/tcmj.tcmj_102_18. Tzu Chi Med J. 2018. PMID: 30305782 Free PMC article. Review. - TREK-king the blood-brain-barrier.
Bittner S, Ruck T, Fernández-Orth J, Meuth SG. Bittner S, et al. J Neuroimmune Pharmacol. 2014 Jun;9(3):293-301. doi: 10.1007/s11481-014-9530-8. Epub 2014 Feb 21. J Neuroimmune Pharmacol. 2014. PMID: 24557892 Review.
References
- Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. - PubMed
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. - PubMed
- Bizzoco E, Lolli F, Repice AM, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol. 2009;256:1891–1898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- P30 DK072517-07/DK/NIDDK NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 EY013574-10/EY/NEI NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- R01 DK035124-23A2/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R37 EB000415-18/EB/NIBIB NIH HHS/United States
- RC1 DK086125-02/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R01 HL073856-08/HL/NHLBI NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources