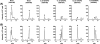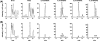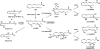Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid - PubMed (original) (raw)
. 2012 Mar 23;287(13):10525-10534.
doi: 10.1074/jbc.M112.340612. Epub 2012 Jan 24.
Makoto Arita 2, Shinnosuke Matsueda 3, Ryo Iwamoto 4, Takuji Fujihara 5, Hiroki Nakanishi 6, Ryo Taguchi 6, Koji Masuda 5, Kenji Sasaki 7, Daisuke Urabe 7, Masayuki Inoue 7, Hiroyuki Arai 3
Affiliations
- PMID: 22275352
- PMCID: PMC3322993
- DOI: 10.1074/jbc.M112.340612
Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid
Yosuke Isobe et al. J Biol Chem. 2012.
Abstract
Bioactive mediators derived from omega-3 eicosapentaenoic acid (EPA) elicit potent anti-inflammatory actions. Here, we identified novel EPA metabolites, including 8,18-dihydroxyeicosapentaenoic acid (8,18-diHEPE), 11,18-diHEPE, 12,18-diHEPE, and 17,18-diHEPE from 18-HEPE. Unlike resolvins E1 and E2, both of which are biosynthesized by neutrophils via the 5-lipoxygenase pathway, these metabolites are biosynthesized by eosinophils via the 12/15-lipoxygenase pathway. Among them, two stereoisomers of 17,18-diHEPE, collectively termed resolvin E3 (RvE3), displayed a potent anti-inflammatory action by limiting neutrophil infiltration in zymosan-induced peritonitis. The planar structure of RvE3 was unambiguously determined to be 17,18-dihydroxy-5Z,8Z,11Z,13E,15E-EPE by high resolution NMR, and the two stereoisomers were assigned to have 17,18R- and 17,18S-dihydroxy groups, respectively, using chemically synthesized 18R- and 18S-HEPE as precursors. Both 18R- and 18S-RvE3 inhibited neutrophil chemotaxis in vitro at low nanomolar concentrations. These findings suggest that RvE3 contributes to the beneficial actions of EPA in controlling inflammation and related diseases.
Figures
FIGURE 1.
Formation of 18-HEPE metabolites from human leukocyte incubations. MRM chromatograms of the 18-HEPE incubation products with human PMN (A) and eosinophils (B) were separated by reverse-phase HPLC. 18-HEPE metabolites were monitored by MRM mode using established transitions for RvE1 (349/195 m/z) and RvE2 (333/199 m/z) as well as predicted transitions for 8,18-diHEPE (333/159 m/z), 11,18-diHEPE (333/167 m/z), 12,18-diHEPE (333/163 m/z), and 17,18-diHEPE (333/201 m/z). Peaks of each metabolite are marked by asterisks.
FIGURE 2.
12/15-LOX-dependent formation of 18-HEPE metabolites from mouse eosinophils. Lipidomic profiles of 18-HEPE incubation products of mouse eosinophils (A) and 12/15-LOX-deficient mouse eosinophils (B) were compared.
FIGURE 3.
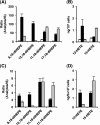
Formation of 18-HEPE metabolites by cells expressing mouse 12/15-LOX or human 15-LOX. Lipidomic profiles of 18-HEPE (A) or arachidonic acid (B) incubation products of HEK293 cells transiently transfected with mock (white bars), mouse 12/15-LOX (black bars), or human 15-LOX (gray bars) cDNA plasmids are shown. Lipidomic profiles of 18-HEPE (C) or arachidonic acid (D) incubation products of mouse (black bars) or human (gray bars) eosinophils are shown. Relative production was determined by calculating peak area ratio of each analyte to deuterium-labeled internal standard (LTB4-d4). Values represent mean ± S.E., n = 3–5.
FIGURE 4.
Enzymatic formation of 18-HEPE metabolites and their anti-inflammatory properties in vivo. A, reverse-phase HPLC chromatogram of the 18-HEPE incubation products with soybean 15-LOX monitored with UV absorbance at 270 nm. B, UV and tandem mass spectra of major products isolated from soybean 15-LOX incubation. Based on the MS/MS spectra, compounds I to IV were assigned as 11,18-diHEPE with corresponding fragments at m/z 333(M-H), 315(M-H-H2O), 297(M-H-2H2O), 271(M-H-H2O-CO2), 253(M-H-2H2O-CO2), and diagnostic fragments at m/z 275, 231(275-CO2), and 167. Compounds V and VI were assigned as 17,18-diHEPE with corresponding fragments at m/z 333(M-H), 315(M-H-H2O), 297(M-H-2H2O), 271(M-H-H2O-CO2), 253(M-H-2H2O-CO2), and diagnostic ions at m/z 275, 257(275-H2O), 245, 213(275-H2O-CO2), and 201(245-CO2).
FIGURE 5.
Comparison of eosinophil-derived 18-HEPE metabolites with enzymatically generated products. Top panel, MRM chromatograms of 11,18-diHEPE (A) and 17,18-diHEPE (B) obtained from eosinophil incubation with 18-HEPE. Lower panels, MRM chromatograms of compounds I–VI obtained from soybean 15-LOX-catalyzed synthesis and co-injection of these enzymatically generated products with eosinophil derived products. Note that compounds II and III co-eluted in this liquid chromatographic condition.
FIGURE 6.
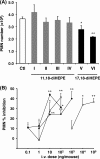
Inhibition of PMN infiltration in zymosan-induced peritonitis. A, compounds I–VI were injected intravenously (10 ng/mouse) via tail vein followed by peritoneal injection of zymosan A (1 mg/ml). After 2 h, peritoneal lavages were collected, and PMN leukocyte numbers were counted. Values represent mean ± S.E., n = 3–12, *, p < 0.05; **, p < 0.01 as compared with vehicle control. B, dose-dependent comparison of the actions of compound V (□), compound VI (■), RvE2 (●) and dexamethasone (○) on PMN infiltration. Values represent mean ± S.E., n = 4–12, *, p < 0.05; **, p < 0.01 as compared with vehicle control.
FIGURE 7.
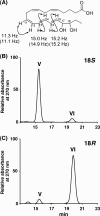
Physical and spectroscopic properties of RvE3. A, 1H-1H coupling constants of conjugated triene and full structure of RvE3 (compound V and VI). B and C, reverse-phase HPLC chromatogram of synthetic 18_S_-HEPE or 18_R_-HEPE incubation products with soybean 15-LOX monitored with UV absorbance at 270 nm.
FIGURE 8.
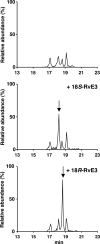
RvE3 formation in vivo. Comparison of endogenously formed 17,18-diHEPEs in mouse inflammatory exudates with enzymatically generated RvE3 isomers. Top panel, MRM chromatogram with established transition of 333/201 m/z to monitor 17,18-diHEPEs present in murine peritoneal exudates 48 h after zymosan challenge. Middle and lower panel, MRM chromatogram obtained from co-injection of enzymatically generated 18_S_-RvE3 (middle panel) or 18_R_-RvE3 (lower panel) with 17,18-diHEPEs present in murine peritoneal exudates 48 h after zymosan challenge.
FIGURE 9.
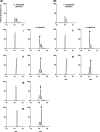
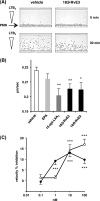
RvE3 reduces mouse PMN chemotaxis efficiency. Effect of RvE3 on chemotaxis of mouse bone marrow PMNs toward LTB4. A, bone marrow PMNs were incubated with 10 n
m
18_S_-RvE3 during the assay. Images of PMNs are shown at 0 and 30 min after addition of LTB4 (10 n
m
) as a chemoattractant. B, velocity of the motile cells were determined from digital time lapse movies. C, concentration dependence of 18_S_-RvE3 (○) and 18_R_-RvE3 (●) on reduced velocity of PMN chemotaxis. Values represent mean ± S.E., n ≥20 cells, *, p < 0.05; **, p < 0.01; ***, p < 0.001 as compared with vehicle control.
FIGURE 10.
Proposed scheme for biosynthesis of E series resolvins and related products. E series resolvins are generated from a common precursor 18-HEPE. EPA is converted to 18-HEPE by aspirin-acetylated COX-2 or cytochrome P450 monooxygenase. 18-HEPE is further converted via the sequential actions of lipoxygenases, which leads to formation of E series resolvins. 5-LOX expressed in PMNs converts 18-HEPE into RvE1 and RvE2. The stereochemistry of RvE1 and RvE2 was established (9, 12). In addition, 18-HEPE is converted by 12/15-LOX present in eosinophils or resident macrophages into 8,18-diHEPE, 11,18-diHEPE, 12,18-diHEPE, and 17,18-diHEPE (RvE3). The stereochemistry of the alcohols in 8,18-diHEPE and 12,18-diHEPE is depicted as tentative.
Similar articles
- Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3.
Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K, Inoue M, Arai H. Isobe Y, et al. J Biochem. 2013 Apr;153(4):355-60. doi: 10.1093/jb/mvs151. Epub 2013 Jan 3. J Biochem. 2013. PMID: 23293324 - Altering 15-Lipoxygenases to 18-Lipoxygenases and Their Application to the Production of 5,18-Dihydroxyeicosapentaenoic Acids.
Lee J, Kang SH, Lee TE, Oh DK. Lee J, et al. Biotechnol Bioeng. 2025 Jul;122(7):1759-1769. doi: 10.1002/bit.28995. Epub 2025 Apr 16. Biotechnol Bioeng. 2025. PMID: 40241317 Free PMC article. - Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions.
Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Serhan CN, et al. J Exp Med. 2009 Jan 16;206(1):15-23. doi: 10.1084/jem.20081880. Epub 2008 Dec 22. J Exp Med. 2009. PMID: 19103881 Free PMC article. - Chiral lipidomics of E-series resolvins: aspirin and the biosynthesis of novel mediators.
Oh SF, Vickery TW, Serhan CN. Oh SF, et al. Biochim Biophys Acta. 2011 Nov;1811(11):737-47. doi: 10.1016/j.bbalip.2011.06.007. Epub 2011 Jun 16. Biochim Biophys Acta. 2011. PMID: 21712098 Free PMC article. Review. - Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers.
Serhan CN, Arita M, Hong S, Gotlinger K. Serhan CN, et al. Lipids. 2004 Nov;39(11):1125-32. doi: 10.1007/s11745-004-1339-7. Lipids. 2004. PMID: 15726828 Review.
Cited by
- Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells.
Jaudszus A, Gruen M, Watzl B, Ness C, Roth A, Lochner A, Barz D, Gabriel H, Rothe M, Jahreis G. Jaudszus A, et al. J Lipid Res. 2013 Apr;54(4):923-35. doi: 10.1194/jlr.P031260. Epub 2013 Jan 24. J Lipid Res. 2013. PMID: 23349208 Free PMC article. - Possibility of averting cytokine storm in SARS-COV 2 patients using specialized pro-resolving lipid mediators.
Yasmeen N, Selvaraj H, Lakhawat SS, Datta M, Sharma PK, Jain A, Khanna R, Srinivasan J, Kumar V. Yasmeen N, et al. Biochem Pharmacol. 2023 Mar;209:115437. doi: 10.1016/j.bcp.2023.115437. Epub 2023 Jan 30. Biochem Pharmacol. 2023. PMID: 36731803 Free PMC article. Review. - Eicosapentaenoic acid prevents the progression of intracranial aneurysms in rats.
Abekura Y, Ono I, Kawashima A, Takizawa K, Koseki H, Miyata H, Shimizu K, Oka M, Kushamae M, Miyamoto S, Kataoka H, Ishii A, Aoki T. Abekura Y, et al. J Neuroinflammation. 2020 Apr 24;17(1):129. doi: 10.1186/s12974-020-01802-8. J Neuroinflammation. 2020. PMID: 32331514 Free PMC article. - Methods of the Analysis of Oxylipins in Biological Samples.
Liakh I, Pakiet A, Sledzinski T, Mika A. Liakh I, et al. Molecules. 2020 Jan 15;25(2):349. doi: 10.3390/molecules25020349. Molecules. 2020. PMID: 31952163 Free PMC article. Review. - Short-term n-3 fatty acid supplementation but not aspirin increases plasma proresolving mediators of inflammation.
Barden A, Mas E, Croft KD, Phillips M, Mori TA. Barden A, et al. J Lipid Res. 2014 Nov;55(11):2401-7. doi: 10.1194/jlr.M045583. Epub 2014 Sep 3. J Lipid Res. 2014. PMID: 25187667 Free PMC article. Clinical Trial.
References
- Nathan C., Ding A. (2010) Nonresolving inflammation. Cell 140, 871–882 - PubMed
- Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. (2004) Inflammatory resolution. New opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 - PubMed
- GISSI-Prevenzione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction. Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455 - PubMed
- Simopoulos A. P. (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21, 495–505 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials