RNA-based therapeutics: current progress and future prospects - PubMed (original) (raw)
Review
RNA-based therapeutics: current progress and future prospects
John C Burnett et al. Chem Biol. 2012.
Abstract
Recent advances of biological drugs have broadened the scope of therapeutic targets for a variety of human diseases. This holds true for dozens of RNA-based therapeutics currently under clinical investigation for diseases ranging from genetic disorders to HIV infection to various cancers. These emerging drugs, which include therapeutic ribozymes, aptamers, and small interfering RNAs (siRNAs), demonstrate the unprecedented versatility of RNA. However, RNA is inherently unstable, potentially immunogenic, and typically requires a delivery vehicle for efficient transport to the targeted cells. These issues have hindered the clinical progress of some RNA-based drugs and have contributed to mixed results in clinical testing. Nevertheless, promising results from recent clinical trials suggest that these barriers may be overcome with improved synthetic delivery carriers and chemical modifications of the RNA therapeutics. This review focuses on the clinical results of siRNA, RNA aptamer, and ribozyme therapeutics and the prospects for future successes.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
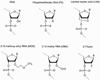
Figure 1
Common chemical modifications of therapeutic nucleic acid analogs. The unmodified RNA structure is shown next to backbone (5’-phosphorothioate), LNA, and 2’-substitutions (2’-O-methoxy-ethyl, 2’-O-methyl, and 2’-fluoro).
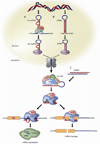
Figure 2
Mammalian PTGS pathway for miRNAs, shRNAs, and siRNAs (A) miRNAs are transcribed from DNA as primary miRNAs (pri-miRNAs) and processed into 70-nt stem-loop precursor miRNAs (pre-miRNAs) by Drosha and DGCR8. The pre-miRNAs are transported to the cytoplasm by dsRNA-binding protein exportin 5, where they are processed into ~22 nt miRNA duplexes by the Dicer/TRBP complex. The imperfectly complementary miRNA duplexes associate with an AGO protein and are loaded into RISC, where the passenger strand is removed and the guide strand remains to target mRNA for silencing. The resulting mature RISC complex may silence gene expression either by inhibiting the initiation of translation or by transporting the complex to cytoplasmic processing bodies (p-bodies) where the mRNA is deadenylated and destroyed. (B) Like miRNAs, shRNAs are transcribed from DNA and undergo similar processing. However, the perfect Watson-Crick base-pairing between the guide strand and the target mRNA triggers AGO2-mediated cleavage of the mRNA target. (C) In contrast to shRNAs, siRNAs are artificially introduced into the cytoplasm. All steps of siRNA and shRNA are the same after processing by Dicer/TRBP.
Similar articles
- Current progress on aptamer-targeted oligonucleotide therapeutics.
Dassie JP, Giangrande PH. Dassie JP, et al. Ther Deliv. 2013 Dec;4(12):1527-46. doi: 10.4155/tde.13.118. Ther Deliv. 2013. PMID: 24304250 Free PMC article. Review. - Prospects for nucleic acid-based therapeutics against hepatitis C virus.
Lee CH, Kim JH, Lee SW. Lee CH, et al. World J Gastroenterol. 2013 Dec 21;19(47):8949-62. doi: 10.3748/wjg.v19.i47.8949. World J Gastroenterol. 2013. PMID: 24379620 Free PMC article. Review. - Current RNA-based Therapeutics in Clinical Trials.
Zhou LY, Qin Z, Zhu YH, He ZY, Xu T. Zhou LY, et al. Curr Gene Ther. 2019;19(3):172-196. doi: 10.2174/1566523219666190719100526. Curr Gene Ther. 2019. PMID: 31566126 Review. - Intracellular delivery of RNA-based therapeutics using aptamers.
Thiel KW, Giangrande PH. Thiel KW, et al. Ther Deliv. 2010 Dec;1(6):849-61. doi: 10.4155/tde.10.61. Ther Deliv. 2010. PMID: 21643487 Free PMC article. Review. - Therapeutic oligonucleotides.
Goodchild J. Goodchild J. Methods Mol Biol. 2011;764:1-15. doi: 10.1007/978-1-61779-188-8_1. Methods Mol Biol. 2011. PMID: 21748630 Review.
Cited by
- Targeting HIV transcription: the quest for a functional cure.
Mousseau G, Mediouni S, Valente ST. Mousseau G, et al. Curr Top Microbiol Immunol. 2015;389:121-45. doi: 10.1007/82_2015_435. Curr Top Microbiol Immunol. 2015. PMID: 25731772 Free PMC article. Review. - Synthetic Polymer Hybridization with DNA and RNA Directs Nanoparticle Loading, Silencing Delivery, and Aptamer Function.
Zhou Z, Xia X, Bong D. Zhou Z, et al. J Am Chem Soc. 2015 Jul 22;137(28):8920-3. doi: 10.1021/jacs.5b05481. Epub 2015 Jul 8. J Am Chem Soc. 2015. PMID: 26138550 Free PMC article. - TREX1 as a Novel Immunotherapeutic Target.
Hemphill WO, Simpson SR, Liu M, Salsbury FR Jr, Hollis T, Grayson JM, Perrino FW. Hemphill WO, et al. Front Immunol. 2021 Apr 1;12:660184. doi: 10.3389/fimmu.2021.660184. eCollection 2021. Front Immunol. 2021. PMID: 33868310 Free PMC article. - RNAi joins the "Singles Club".
Peschansky VJ, Liang Z, Wahlestedt C. Peschansky VJ, et al. Mol Ther. 2012 Nov;20(11):2010-1. doi: 10.1038/mt.2012.218. Mol Ther. 2012. PMID: 23131851 Free PMC article. No abstract available. - Biomedical Applications of RNA-Based Devices.
Kim CM, Smolke CD. Kim CM, et al. Curr Opin Biomed Eng. 2017 Dec;4:106-115. doi: 10.1016/j.cobme.2017.10.005. Epub 2017 Oct 18. Curr Opin Biomed Eng. 2017. PMID: 36237555 Free PMC article.
References
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. - PubMed
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. - PubMed
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
- Melnikova I. RNA-based therapies. Nature Reviews Drug Discovery. 2007;6:863–864. - PubMed
- Peer D, Lieberman J. Special delivery: targeted therapy with small RNAs. Gene Ther. 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI042552-15/AI/NIAID NIH HHS/United States
- R37 AI029329-23/AI/NIAID NIH HHS/United States
- AI29329/AI/NIAID NIH HHS/United States
- AI42552/AI/NIAID NIH HHS/United States
- R37 AI029329/AI/NIAID NIH HHS/United States
- HL07470/HL/NHLBI NIH HHS/United States
- R01 AI042552/AI/NIAID NIH HHS/United States
- R01 AI029329/AI/NIAID NIH HHS/United States
- R01 AI029329-08S1/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources