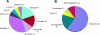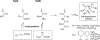Lessons from the past and charting the future of marine natural products drug discovery and chemical biology - PubMed (original) (raw)
Review
Lessons from the past and charting the future of marine natural products drug discovery and chemical biology
William H Gerwick et al. Chem Biol. 2012.
Erratum in
- Chem Biol. 2012 Dec 21;19(12):1631
Abstract
Marine life forms are an important source of structurally diverse and biologically active secondary metabolites, several of which have inspired the development of new classes of therapeutic agents. These success stories have had to overcome difficulties inherent to natural products-derived drugs, such as adequate sourcing of the agent and issues related to structural complexity. Nevertheless, several marine-derived agents are now approved, most as "first-in-class" drugs, with five of seven appearing in the past few years. Additionally, there is a rich pipeline of clinical and preclinical marine compounds to suggest their continued application in human medicine. Understanding of how these agents are biosynthetically assembled has accelerated in recent years, especially through interdisciplinary approaches, and innovative manipulations and re-engineering of some of these gene clusters are yielding novel agents of enhanced pharmaceutical properties compared with the natural product.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
A) Pie chart illustrating the original collected sources of marine natural product derived or inspired agents currently as approved drugs or in clinical trials (20 total). B) Pie chart of the marine-derived drugs and clinical trial agents divided by their subsequently shown or predicted source organisms (20 total).
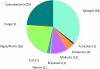
Figure 2
Pie chart illustrating the collected sources of marine natural products that are available commercially for their useful pharmacological properties in biomedical research (121 total).
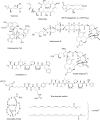
Figure 3
Chemical structures of the approved drugs deriving from or inspired by a marine natural product and other marine metabolites discussed in the text (one letter amino acid codes are used for depicting the structure of ziconotide).
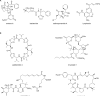
Figure 4
Examples of marine natural products from (A) laboratory cultured and (B) environmental uncultured marine microbes whose biosynthetic pathways have been established by a variety of omic approaches (includes ecteinascidin-743 shown in Figure 3).
Figure 5
Assembly line biosynthesis of salinosporamide and library development of structure analogs via mutasynthesis and other genetic engineering approaches.
Figure 6
Parallel strategy employed by Grindberg et al. (2011) to rapidly access the biosynthetic gene cluster for apratoxin A, a promising anticancer lead compound from the marine cyanobacterium Moorea bouillonii. On the top arm, single cells are obtained by microdissection from non-axenic cultures of cyanobacteria, and DNA is extracted and amplified by Multiple Displacement Amplification (MDA) for partial genome sequencing. The sequences of recognizable gene motifs associated with natural product pathways are then used to construct PCR probes to screen a fosmid library which is produced in the normal fashion (lower arm). Fosmids probing positively by this process can be further characterized for desired gene motifs, and then sequenced. The melding of these approaches can accelerate the process of biosynthetic gene cluster discovery and description, such as is illustrated here for apratoxin A, especially in cases of non-axenic cultures or environmental samples.
Similar articles
- Medicinal Purposes: Bioactive Metabolites from Marine-derived Organisms.
Li T, Ding T, Li J. Li T, et al. Mini Rev Med Chem. 2019;19(2):138-164. doi: 10.2174/1389557517666170927113143. Mini Rev Med Chem. 2019. PMID: 28969543 Review. - Marine Pharmacology in 2016-2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action.
Mayer AMS, Guerrero AJ, Rodríguez AD, Taglialatela-Scafati O, Nakamura F, Fusetani N. Mayer AMS, et al. Mar Drugs. 2021 Jan 21;19(2):49. doi: 10.3390/md19020049. Mar Drugs. 2021. PMID: 33494402 Free PMC article. Review. - An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs.
Ruiz-Torres V, Encinar JA, Herranz-López M, Pérez-Sánchez A, Galiano V, Barrajón-Catalán E, Micol V. Ruiz-Torres V, et al. Molecules. 2017 Jun 23;22(7):1037. doi: 10.3390/molecules22071037. Molecules. 2017. PMID: 28644406 Free PMC article. Review. - A Comprehensive Review of Bioactive Peptides from Marine Fungi and Their Biological Significance.
Youssef FS, Ashour ML, Singab ANB, Wink M. Youssef FS, et al. Mar Drugs. 2019 Sep 29;17(10):559. doi: 10.3390/md17100559. Mar Drugs. 2019. PMID: 31569458 Free PMC article. Review. - Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification.
Jing Q, Hu X, Ma Y, Mu J, Liu W, Xu F, Li Z, Bai J, Hua H, Li D. Jing Q, et al. Mar Drugs. 2019 Jun 27;17(7):384. doi: 10.3390/md17070384. Mar Drugs. 2019. PMID: 31252563 Free PMC article. Review.
Cited by
- Natural products: a continuing source of novel drug leads.
Cragg GM, Newman DJ. Cragg GM, et al. Biochim Biophys Acta. 2013 Jun;1830(6):3670-95. doi: 10.1016/j.bbagen.2013.02.008. Epub 2013 Feb 18. Biochim Biophys Acta. 2013. PMID: 23428572 Free PMC article. Review. - Some Biogenetic Considerations Regarding the Marine Natural Product (-)-Mucosin.
Nolsøe JMJ, Aursnes M, Stenstrøm YH, Hansen TV. Nolsøe JMJ, et al. Molecules. 2019 Nov 15;24(22):4147. doi: 10.3390/molecules24224147. Molecules. 2019. PMID: 31731797 Free PMC article. - Chemical Ecology of Marine Sponges: New Opportunities through "-Omics".
Paul VJ, Freeman CJ, Agarwal V. Paul VJ, et al. Integr Comp Biol. 2019 Oct 1;59(4):765-776. doi: 10.1093/icb/icz014. Integr Comp Biol. 2019. PMID: 30942859 Free PMC article. Review. - Lactomycins A-C, Dephosphorylated Phoslactomycin Derivatives that Inhibit Cathepsin B, from the Marine-derived Streptomyces sp. ACT232.
Sun Y, Carandang RR, Harada Y, Okada S, Yoshitake K, Asakawa S, Nogi Y, Matsunaga S, Takada K. Sun Y, et al. Mar Drugs. 2018 Feb 21;16(2):70. doi: 10.3390/md16020070. Mar Drugs. 2018. PMID: 29466301 Free PMC article. - Choose Your Weaponry: Selective Storage of a Single Toxic Compound, Latrunculin A, by Closely Related Nudibranch Molluscs.
Cheney KL, White A, Mudianta IW, Winters AE, Quezada M, Capon RJ, Mollo E, Garson MJ. Cheney KL, et al. PLoS One. 2016 Jan 20;11(1):e0145134. doi: 10.1371/journal.pone.0145134. eCollection 2016. PLoS One. 2016. PMID: 26788920 Free PMC article.
References
- Abel U, Koch C, Speitling M, Hansske FG. Modern methods to produce natural-product libraries. Curr. Op. Chem. Biol. 2002;6:453–458. - PubMed
- Bergmann W, Watkins JC, Stempien MF., Jr. Contribution to the study of marine products. XLV. Sponge nucleic acids. J. Org. Chem. 1957;22:1308–13.
- Borissenko L, Groll M. 20S proteasome and its inhibitors: Crystallographic knowledge for drug development. Chem. Rev. 2007;107:687–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA100851/CA/NCI NIH HHS/United States
- CA127622/CA/NCI NIH HHS/United States
- TW006634/TW/FIC NIH HHS/United States
- GM857770/GM/NIGMS NIH HHS/United States
- R01 GM085770-04/GM/NIGMS NIH HHS/United States
- AI47818/AI/NIAID NIH HHS/United States
- U01 TW006634/TW/FIC NIH HHS/United States
- R01 CA108874/CA/NCI NIH HHS/United States
- CA108874/CA/NCI NIH HHS/United States
- R01 CA127622/CA/NCI NIH HHS/United States
- R01 NS053398/NS/NINDS NIH HHS/United States
- CA100851/CA/NCI NIH HHS/United States
- R01 CA108874-07/CA/NCI NIH HHS/United States
- R01 CA127622-05/CA/NCI NIH HHS/United States
- R01 NS053398-11/NS/NINDS NIH HHS/United States
- U01 TW006634-10/TW/FIC NIH HHS/United States
- R56 AI047818/AI/NIAID NIH HHS/United States
- NS053398/NS/NINDS NIH HHS/United States
- R01 AI047818-13/AI/NIAID NIH HHS/United States
- R01 GM085770/GM/NIGMS NIH HHS/United States
- R01 AI047818/AI/NIAID NIH HHS/United States
- R01 CA100851-09/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous