Proteasome inhibitors: an expanding army attacking a unique target - PubMed (original) (raw)
Review
Proteasome inhibitors: an expanding army attacking a unique target
Alexei F Kisselev et al. Chem Biol. 2012.
Abstract
Proteasomes are large, multisubunit proteolytic complexes presenting multiple targets for therapeutic intervention. The 26S proteasome consists of a 20S proteolytic core and one or two 19S regulatory particles. The 20S core contains three types of active sites. Many structurally diverse inhibitors of these active sites, both natural product and synthetic, have been discovered in the last two decades. One, bortezomib, is used clinically for treatment of multiple myeloma, mantle cell lymphoma, and acute allograft rejection. Five more recently developed proteasome inhibitors are in trials for treatment of myeloma and other cancers. Proteasome inhibitors also have activity in animal models of autoimmune and inflammatory diseases, reperfusion injury, promote bone and hair growth, and can potentially be used as anti-infectives. In addition, inhibitors of ATPases and deubiquitinases of 19S regulatory particles have been discovered in the last decade.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
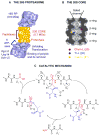
Figure 1. The Proteasome
(A) The 26S particle. Location and functions of different subunits are indicated. (B) Cross-section of the 20S proteolytic core showing location of the active sites. (C) The catalytic mechanism of the proteasome. Proteasome is blue. Substrate is black except for scissile bond, which is red.
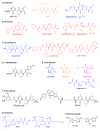
Figure 2. Representatives of the Major Classes of Covalent Proteasome Inhibitors
(A) Aldehydes; (B) boronates; (C) epoxyketones; (D) α-ketoaldehyde; (E) β-lactones; (F) vinyl-sulfones; (G) syrbactines; (H) bacteria-specific oxatiazol-2-ones. Natural products are blue. Synthetic inhibitors used clinically for the treatment of cancer (FDA-approved or in clinical trials) are red; natural product in clinical trials for the treatment of cancer is purple. Synthetic inhibitors that were tested clinically for other indications are orange. (Omuralide is a derivative of a natural product lactacystin.)
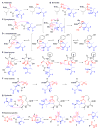
Figure 3. Mechanism of Proteasome Inhibition by Covalent Inhibitors
(A) Aldehydes; (B) boronates; (C) epoxyketones; (D) α-ketoaldehyde; (E) β-lactones; (F) vinyl-sulfones; (G) syrbactines; (H) bacteria-specific oxatiazol-2-ones. Proteasome is blue. Inhibitors are black except for electrophiles, which are red.
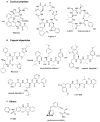
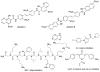
Figure 4. Noncovalent Proteasome Inhibitors
(A) Cyclic peptides. (B) N- and C-terminally capped dipeptides. (C) Others.
Figure 5. Site-Specific Inhibitors
(A) Inhibitors of the chymotrypsin-like sites: YU-101 (Elofsson et al., 1999), NC-005 (Britton et al., 2009), NC-005-VS (Screen et al., 2010), and LU-005 (Geurink et al., 2010). (B) Inhibitors of the caspase-like sites. YU-102 (Myung et al., 2001), NC-001 (Britton et al., 2009), and LU-001 (van der Linden et al., 2012) inhibit β1 and β1i sites. (C) Inhibitor of the trypsin-like sites (Mirabella et al., 2011). (D) Inhibitors with selectivity for immunoproteasome subunits over their constitutive counterparts and vice versa. PR-957 (Muchamuel et al., 2009) is β5i (LMP7)-selective, and CPSI (Parlati et al., 2009) is β5-selective. LMP2-sp-ek (Ho et al., 2007) and IPSI-001 (Kuhn et al., 2009) are β1i (LMP2)-selective. (E) Activity-based probes (Mirabella et al., 2011; Verdoes et al., 2010). Azido-NC-002 requires subsequent modification by a biotinylated phospane in a Staudinger-Bertozzi ligation to reveal polypeptides modified by the probe.
Figure 6
Inhibitors of 19S RP
Similar articles
- Inhibition of proteasome deubiquitinating activity as a new cancer therapy.
D'Arcy P, Brnjic S, Olofsson MH, Fryknäs M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S. D'Arcy P, et al. Nat Med. 2011 Nov 6;17(12):1636-40. doi: 10.1038/nm.2536. Nat Med. 2011. PMID: 22057347 - Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib.
Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, Verdoes M, Filippov DV, Overkleeft HS, Kisselev AF. Mirabella AC, et al. Chem Biol. 2011 May 27;18(5):608-18. doi: 10.1016/j.chembiol.2011.02.015. Chem Biol. 2011. PMID: 21609842 Free PMC article. - Novel proteasome inhibitors to overcome bortezomib resistance.
Ruschak AM, Slassi M, Kay LE, Schimmer AD. Ruschak AM, et al. J Natl Cancer Inst. 2011 Jul 6;103(13):1007-17. doi: 10.1093/jnci/djr160. Epub 2011 May 23. J Natl Cancer Inst. 2011. PMID: 21606441 Review. - Proteasome inhibitors in the clinical setting: benefits and strategies to overcome multiple myeloma resistance to proteasome inhibitors.
Cheriyath V, Jacobs BS, Hussein MA. Cheriyath V, et al. Drugs R D. 2007;8(1):1-12. doi: 10.2165/00126839-200708010-00001. Drugs R D. 2007. PMID: 17249845 Review. - Ubiquitin and cancer: from molecular targets and mechanisms to the clinic -- AACR Special Conference.
Colland F. Colland F. IDrugs. 2006 Mar;9(3):179-81. IDrugs. 2006. PMID: 16523381 No abstract available.
Cited by
- Cryptic enzymatic assembly of peptides armed with β-lactone warheads.
Xu G, Torri D, Cuesta-Hoyos S, Panda D, Yates LRL, Zallot R, Bian K, Jia D, Iorgu AI, Levy C, Shepherd SA, Micklefield J. Xu G, et al. Nat Chem Biol. 2024 Oct;20(10):1371-1379. doi: 10.1038/s41589-024-01657-7. Epub 2024 Jul 1. Nat Chem Biol. 2024. PMID: 38951647 Free PMC article. - SCFFBXW11 Complex Targets Interleukin-17 Receptor A for Ubiquitin-Proteasome-Mediated Degradation.
Jin B, Moududee SA, Ge D, Zhou P, Wang AR, Liu YZ, You Z. Jin B, et al. Biomedicines. 2024 Mar 28;12(4):755. doi: 10.3390/biomedicines12040755. Biomedicines. 2024. PMID: 38672111 Free PMC article. - Macrocyclic Oxindole Peptide Epoxyketones-A Comparative Study of Macrocyclic Inhibitors of the 20S Proteasome.
Götz MG, Godwin K, Price R, Dorn R, Merrill-Steskal G, Klemmer W, Hansen H, Produturi G, Rocha M, Palmer M, Molacek L, Strater Z, Groll M. Götz MG, et al. ACS Med Chem Lett. 2024 Mar 27;15(4):533-539. doi: 10.1021/acsmedchemlett.4c00017. eCollection 2024 Apr 11. ACS Med Chem Lett. 2024. PMID: 38628795 Free PMC article. - Early recovery of proteasome activity in cells pulse-treated with proteasome inhibitors is independent of DDI2.
Ibtisam I, Kisselev AF. Ibtisam I, et al. Elife. 2024 Apr 15;12:RP91678. doi: 10.7554/eLife.91678. Elife. 2024. PMID: 38619391 Free PMC article. - The nociceptive activity of peripheral sensory neurons is modulated by the neuronal membrane proteasome.
Villalón Landeros E, Kho SC, Church TR, Brennan A, Türker F, Delannoy M, Caterina MJ, Margolis SS. Villalón Landeros E, et al. Cell Rep. 2024 Apr 23;43(4):114058. doi: 10.1016/j.celrep.2024.114058. Epub 2024 Apr 12. Cell Rep. 2024. PMID: 38614084 Free PMC article.
References
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. - PubMed
- Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8:333–338. - PubMed
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. - PubMed
- Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. - PubMed
- Altun M, Galardy PJ, Shringarpure R, Hideshima T, LeBlanc R, Anderson KC, Ploegh HL, Kessler BM. Effects of PS-341 on the activity and composition of proteasomes in multiple myeloma cells. Cancer Res. 2005;65:7896–7901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources