Sulindac inhibits tumor cell invasion by suppressing NF-κB-mediated transcription of microRNAs - PubMed (original) (raw)
Sulindac inhibits tumor cell invasion by suppressing NF-κB-mediated transcription of microRNAs
X Li et al. Oncogene. 2012.
Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) have been widely reported to display strong efficacy for cancer chemoprevention, although their mechanism of action is poorly understood. The most well-documented effects of NSAIDs include inhibition of tumor cell proliferation and induction of apoptosis, but their effect on tumor cell invasion has not been well studied. Here, we show that the NSAID, sulindac sulfide (SS) can potently inhibit the invasion of human MDA-MB-231 breast and HCT116 colon tumor cells in vitro at concentrations less than those required to inhibit tumor cell growth. To study the molecular basis for this activity, we investigated the involvement of microRNA (miRNA). A total of 132 miRNAs were found to be altered in response to SS treatment, including miR-10b, miR-17, miR-21 and miR-9, which have been previously implicated in tumor invasion and metastasis. We confirmed that these miRNA can stimulate tumor cell invasion and show that SS can attenuate their invasive effects by downregulating their expression. Employing luciferase and chromatin immunoprecipitation assays, NF-κB was found to bind the promoters of all four miRNAs to suppress their expression at the transcriptional level. We show that SS can inhibit the translocation of NF-κB to the nucleus by decreasing the phosphorylation of IKKβ and IκB. Analysis of the promoter sequences of the miRNAs suppressed by SS revealed that 81 of 115 sequences contained NF-κB-binding sites. These results show that SS can inhibit tumor cell invasion by suppressing NF-κB-mediated transcription of miRNAs.
Conflict of interest statement
Conflict of Interest: Authors declare there are no competing financial interests in relation to the work described.
Figures
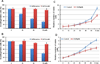
Figure 1. SS inhibits tumor cell invasion without affecting tumor cell growth
(A) MDA-MB-231 and (B) HCT116 cells were treated with SS at 0, 30 µM, 40 µM, and 50 µM for 36 h. The inhibition of cell invasion by SS was dose dependent, and 50 µM had a significant effect on both MDA-MB-231 and HCT116 cells (p<0.05). The same condition did not significantly affect cell growth (p>0.05). (C) MDA-MB-231 and (D) HCT116 cells were treated with SS at 50 µM, and cell growth was determined after various treatment times. The viability of both tumor cell lines was not significantly influenced until 48 h of treatment (p<0.05). (T-test was used for determining statistical significance; * indicates p<0.05)
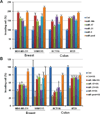
Figure 2. MiRNAs mediate the SS-mediated inhibition of cancer cell invasion
Over-expression of miR-10b, miR-17, miR-21, and miR-9 by transfection of their mimics not only can promote cell invasion in human breast cancer MDA-MB-231 and SUM1315 cells and human colon cancer HCT116 and HT29 cells (A), but also attenuate the inhibitory effect of SS on invasion of tumor cells (B). (T-test was used for determining statistical significance; * indicates p<0.05; ** indicates p<0.01; the error bars represent the standard deviation)
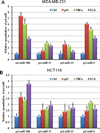
Figure 3. Induced NF-κB regulates the expression of the selected miRNAs at the transcriptional level
After being exposed to 25ng/ml TNFα for 5h or 250µM DCA for 2h, non-treated control and treated MDA-MB-231 and HCT116 cells were harvested for RNA isolation. QRT-PCR was employed for examine the relative expression of pri-miR-10b, pri-miR-17, pri-miR-21, and pri-miR-9. A p65 construct was transiently transfected into MDA-MB-231 and HCT116 cells as a positive control for NF-κB over-expression. (T-test was used for determining statistical significance; * indicates p<0.05; ** indicates p<0.01; the error bars represent the standard deviation)
Figure 4. NF-κB directly binds to the selected miRNAs promoters
(A) Schematic of miR-10b promoter fragments containing p65 NF-κB binding sites. DNA fragments including two putative binding sequences of p65 (W1: −1078 to −1065; W2: −379 to −365) and the corresponding mutated sequences (M1 and M2) were cloned. (B) TNFα can induce the relative luciferase activity through W2 (p<0.05) but not W1. (C) Transfection of a p65 NF-κB construct increases the relative luciferase activity via W2 (p<0.05) but not W1 (RLU means relative luminescence units; T-test was used to determine statistical significance; * indicates p<0.05; the error bars represent the standard deviation). (D) ChIP assay of chromatin isolated from HCT116 cells treated with 25 ng/ml TNFα for 20 min and immunoprecipitated by anti-p65 or control IgG, followed by PCR analysis with primers targeted to the upstream sequence (299bp) of the IκBα promoter (the positive control from the kit), or to the sequences including W2 (233bp) and W1 (290bp) at the 5′-end of miR-10b, or to the binding sequence (202bp) in the miR-9-1 promoter. MiR-17 and miR-21 were tested using the previously published primer sequences .
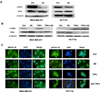
Figure 5. SS prevents the translocation of NF-κB through inhibiting the phosphorylation of IKKβ and IκB
(A)The Western blot assay showed the phosphorylation of IKKβ is decreased in both MDA-MB-231 and HCT116 cells in response to SS treatment. (B) The Western blot assay showed that the decline of phosphorylated IκBα versus the accumulation of IκBα when MDA-MB-231 and HCT116 cells were treated by SS. TNFα (25 ng/ml for 20 min) was used to stimulated the expression of nuclear NF-κB. (C) NF-κB immunofluorescence of MDA-MB-231 and HCT116 cells. The conditions were as follows: (1) control; (2) treatment with 50 µM SS for 12 h; (3) treatment with 25 ng/ml TNFα for 20 min; and (4) both TNFα and SS treatments. The green (anti-NF-κB) indicates NF-κB distribution, and blue indicates the location of the nucleus.
Similar articles
- Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis.
Song L, Liu D, Zhao Y, He J, Kang H, Dai Z, Wang X, Zhang S, Zan Y. Song L, et al. Biochem Biophys Res Commun. 2015 Aug 28;464(3):705-10. doi: 10.1016/j.bbrc.2015.07.004. Epub 2015 Jul 10. Biochem Biophys Res Commun. 2015. PMID: 26166821 - Sulindac activates NF-κB signaling in colon cancer cells.
Mladenova D, Pangon L, Currey N, Ng I, Musgrove EA, Grey ST, Kohonen-Corish MR. Mladenova D, et al. Cell Commun Signal. 2013 Oct 1;11:73. doi: 10.1186/1478-811X-11-73. Cell Commun Signal. 2013. PMID: 24083678 Free PMC article. - Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition.
Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, Piazza GA. Tinsley HN, et al. Cancer Prev Res (Phila). 2010 Oct;3(10):1303-13. doi: 10.1158/1940-6207.CAPR-10-0030. Epub 2010 Sep 28. Cancer Prev Res (Phila). 2010. PMID: 20876730 Free PMC article. - Sulindac inhibits activation of the NF-kappaB pathway.
Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Yamamoto Y, et al. J Biol Chem. 1999 Sep 17;274(38):27307-14. doi: 10.1074/jbc.274.38.27307. J Biol Chem. 1999. PMID: 10480951 - Regulation of the MIR155 host gene in physiological and pathological processes.
Elton TS, Selemon H, Elton SM, Parinandi NL. Elton TS, et al. Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
- Network-based identification of microRNAs as potential pharmacogenomic biomarkers for anticancer drugs.
Li J, Lei K, Wu Z, Li W, Liu G, Liu J, Cheng F, Tang Y. Li J, et al. Oncotarget. 2016 Jul 19;7(29):45584-45596. doi: 10.18632/oncotarget.10052. Oncotarget. 2016. PMID: 27329603 Free PMC article. - The fundamental role of miR-10b in metastatic cancer.
Sheedy P, Medarova Z. Sheedy P, et al. Am J Cancer Res. 2018 Sep 1;8(9):1674-1688. eCollection 2018. Am J Cancer Res. 2018. PMID: 30323962 Free PMC article. Review. - Upregulation of miR-335-3p by NF-κB Transcriptional Regulation Contributes to the Induction of Pulmonary Arterial Hypertension via APJ during Hypoxia.
Fan J, Fan X, Guang H, Shan X, Tian Q, Zhang F, Chen R, Ye F, Quan H, Zhang H, Ding L, Gan Z, Xue F, Wang Y, Mao S, Hu L, Gong Y. Fan J, et al. Int J Biol Sci. 2020 Jan 1;16(3):515-528. doi: 10.7150/ijbs.34517. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015687 Free PMC article. - New use for an old drug: COX-independent anti-inflammatory effects of sulindac in models of cystic fibrosis.
Rocca J, Manin S, Hulin A, Aissat A, Verbecq-Morlot W, Prulière-Escabasse V, Wohlhuter-Haddad A, Epaud R, Fanen P, Tarze A. Rocca J, et al. Br J Pharmacol. 2016 Jun;173(11):1728-41. doi: 10.1111/bph.13464. Epub 2016 Apr 21. Br J Pharmacol. 2016. PMID: 26894321 Free PMC article. - CRISPR/Cas9 ablating viral microRNA promotes lytic reactivation of Kaposi's sarcoma-associated herpesvirus.
Liang Z, Qin Z, Riker AI, Xi Y. Liang Z, et al. Biochem Biophys Res Commun. 2020 Dec 17;533(4):1400-1405. doi: 10.1016/j.bbrc.2020.10.030. Epub 2020 Oct 19. Biochem Biophys Res Commun. 2020. PMID: 33092788 Free PMC article.
References
- Smalley W, Ray WA, Daugherty J. Griffin MR Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer: a population-based study. Arch Intern Med. 1999;159:161–166. - PubMed
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. - PubMed
- Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, Vogelstein B, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. - PubMed
- Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, et al. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis. 1998;19:87–91. - PubMed
- Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–2915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA148817/CA/NCI NIH HHS/United States
- R01 CA155638/CA/NCI NIH HHS/United States
- R21 CA160280/CA/NCI NIH HHS/United States
- R01 CA131378/CA/NCI NIH HHS/United States
- R01 CA140472/CA/NCI NIH HHS/United States
- R01CA148817/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous