Tension is required but not sufficient for focal adhesion maturation without a stress fiber template - PubMed (original) (raw)
Tension is required but not sufficient for focal adhesion maturation without a stress fiber template
Patrick W Oakes et al. J Cell Biol. 2012.
Abstract
Focal adhesion composition and size are modulated in a myosin II-dependent maturation process that controls adhesion, migration, and matrix remodeling. As myosin II activity drives stress fiber assembly and enhanced tension at adhesions simultaneously, the extent to which adhesion maturation is driven by tension or altered actin architecture is unknown. We show that perturbations to formin and α-actinin 1 activity selectively inhibited stress fiber assembly at adhesions but retained a contractile lamella that generated large tension on adhesions. Despite relatively unperturbed adhesion dynamics and force transmission, impaired stress fiber assembly impeded focal adhesion compositional maturation and fibronectin remodeling. Finally, we show that compositional maturation of focal adhesions could occur even when myosin II-dependent cellular tension was reduced by 80%. We propose that stress fiber assembly at the adhesion site serves as a structural template that facilitates adhesion maturation over a wide range of tensions. This work identifies the essential role of lamellar actin architecture in adhesion maturation.
© 2012 Oakes et al.
Figures
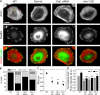
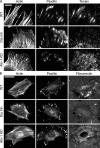
Figure 1.
RSF formation is suppressed through formin inhibition or reduced expression of Dia1 or Atn-1. (A) Images of F-actin visualized by fluorescent phalloidin (top row) and Pxn immunofluorescence (middle row) are shown for WT U2OS cells, cells treated with 15 µM of the formin inhibitor SMIFH2 (Dia Inh), cells with reduced expression of Dia1 via siRNA (Dia1 siRNA), and cells with reduced expression of Atn-1 by shRNA (Atn-1 KD). Yellow arrows indicate RSFs, and red arrows indicate transverse arcs in the WT cells. Bar, 20 µm. (B) Percentages of cells containing both RSFs and transverse arcs (RSF + TA), transverse arcs only (TA Only), or no actin bundles (None) were determined for the WT, Dia Inh, Dia1 siRNA, and Atn-1 KD cells, as described above (n = 49, 50, 22, and 50 for the four conditions, respectively). Representative images from each classification of cells can be seen in
Fig. S3
. (C) Mean linear density of RSFs, measured as the number of RSFs per micrometer along the cell edge for each of the four conditions (error bars indicate SEM; n = at least 10 cells containing five to six RSFs; **, P < 0.01 with respect to WT). (D) Mean background-corrected fluorescence (Fluor.) intensity of fluorescent phalloidin staining of F-actin in transverse arcs and RSFs for each condition, normalized to the transverse arcs in WT cells (error bars indicate SEM; n = 20 regions from 10 cells for each condition; **, P < 0.01; ***, P < 0.001 with respect to WT). A.U., arbitrary unit.
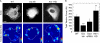
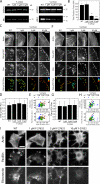
Figure 2.
Suppressing RSF assembly reduces the quantity of active myosin II but increases lamellar actin retrograde flow rate. (A) Immunofluorescence images of pMLC and fluorescent phalloidin staining of F-actin are shown for WT, Dia Inh, and Atn-1 KD U2OS cells. Bar, 20 µm. (B) Western blots showing MLC and pMLC for each condition in relation to a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) loading control. (C) Densitometry analysis of Western blots showing the relative levels of pMLC, MLC, and the ratio of pMLC to MLC for each condition, normalized to WT cells. There is no significant difference in the ratio of MLC to pMLC for each condition. The bar plot shows the mean ratio relative to WT cells (error bars indicate SEM; n = 6 for each condition). (D) A series of images of GFP-actin expressed in a protruding Atn-1 KD cell over time. Time is indicated in minutes/seconds. Bar, 10 µm. See
Video 1
. (E) Magnified images of the boxed region indicated at time 0 in D, illustrating the reorientation of a transverse arc to align in the direction of retrograde flow (red arrowheads). Bar, 2 µm. (F) Actin flow vectors are overlaid on the GFP-actin image from E at 4 min and 30 s. The white arrow represents 15 nm/s. Bar, 10 µm. (G) A box plot of the lamellar retrograde flow speed for each condition described (open circle = mean; box = 25th, 50th, and 75th percentile; whiskers = 5th and 95th percentile; n > 150 flow vectors from multiple regions in three to five cells for each condition; ***, P < 0.001 with respect to WT).
Figure 3.
Cells lacking RSFs generate large traction stresses. (A) Images of GFP-actin (top row) and heat map of traction stress magnitude (bottom row) of WT, Dia Inh, and Atn-1 KD U2OS cells plated on fibronectin-coated PAA gels. For traction stress images, the white line indicates the cell outline, and the heat scale of traction stress (in Pa) is indicated at the bottom right. Bar, 10 µm. (B) Plot of the total traction force exerted by WT, Dia-inhibited, Dia1 siRNA, and Atn-1 KD U2OS cells (error bars indicate SEM; n = 18, 14, 11, and 24 cells for each condition, respectively; *, P < 0.05; **, P < 0.01; ***, P < 0.001 with respect to WT).
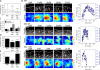
Figure 4.
Substantial tension is built up at focal adhesions despite impaired RSF assembly. (A) Box plots of focal adhesion (FA) lengths of WT, Dia Inh, and Atn-1 KD U2OS cells (open circle = mean; box = 25th, 50th, and 75th percentile; whiskers = 5th and 95th percentile; n > 1,000 from 10 cells for each condition; ***, P < 0.001 with respect to WT). (B) Time series of GFP-Pxn and traction stress heat map images for representative WT, Dia-inhibited, and Atn-1 KD cells, showing the assembly and disassembly of a representative single focal adhesion and changes in traction stress. Bars, 3 µm. Focal adhesion length and normalized traction stress at the focal adhesion indicated by the red ovals are shown on the right. (C) Bar graph of the mean rates of focal adhesion assembly and disassembly for each of three conditions (error bars indicate SEM; n = 10 adhesions from two cells for each condition). (D) Bar graph of the mean focal adhesion lifetime (error bars indicate SEM; n = 26, 24, and 22 from two WT, Dia Inh, and Atn-1 KD cells, respectively; ***, P < 0.001 with respect to WT). (E) Plot of the mean traction stress exerted at individual focal adhesions (error bars indicate SEM; n = 51, 69, and 29 focal adhesions from three cells for WT, Dia-inhibited, and Atn-1 KD U2OS cells, respectively; ***, P < 0.001 with respect to WT).
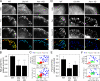
Figure 5.
Reduced accumulation of phosphorylated FAK and Pxn in focal adhesions of cells with impaired RSF assembly. (A) Immunolocalization of F-actin, Pxn, and phosphorylated FAK (FAK-pY397) in representative WT, Dia Inh, and Atn-1 KD U2OS cells. The bottom row shows images representing the ratio of background-subtracted ratio intensity of FAK-pY397 to Pxn at individual focal adhesions in each of the three conditions. The range of the ratio is indicated by the bottom-right scale (in arbitrary units). Bar, 5 µm. (B) Bar graph of the mean ratio of FAK-pY397:Pxn, normalized to the WT cells (error bars indicate SEM; n > 1,000 focal adhesions from 10 cells for each condition; ***, P < 0.001 with respect to WT). (C) The mean intensity (Inten.) of FAK-pY397 or Pxn plotted against the mean intensity of actin at each focal adhesion (FA) for the focal adhesions shown in the bottom row of A. A.U., arbitrary unit. (D) Immunolocalization of actin, Pxn, and phosphorylated Pxn (Pxn-pY31) for each condition. The bottom row of images indicates the ratio of Pxn-pY31 to Pxn for each condition described. The range of the ratio is indicated by the bottom-right scale (in arbitrary units). Bar, 5 µm. (E) Bar graph of the mean ratio of Pxn-pY31:Pxn, normalized to the WT cells (error bars indicate SEM; _n_ > 700 focal adhesions from six cells for each condition; **, P < 0.01 with respect to WT). (F) The mean intensity of Pxn-pY31 or Pxn plotted against the mean intensity of actin at each focal adhesion for the focal adhesions shown in the bottom row of D.
Figure 6.
The maturation of focal adhesions into tensin-rich fibrillar adhesions is abrogated in the absence of RSFs. (A) Representative immunofluorescence TIRF images of Pxn and tensin along with the corresponding confocal actin image for WT, Dia Inh, and Atn-1 KD fibroblasts on fibronectin-coated glass coverslips. Bar, 10 µm. (B) Representative immunofluorescence actin, Pxn, and fibronectin images for WT, Dia-inhibited, and Atn-1 KD fibroblasts on fibronectin-coated glass coverslips 24 h after plating. The arrows indicate representative areas of fibronectin remodeling. Bar, 50 µm.
Figure 7.
Composition maturation of focal adhesions and matrix remodeling occurs when cellular tension is reduced by 80%. (A) Western blot showing reduced levels of pMLC in U2OS cells in the presence of 1, 5, and 10 µM Y-27632 in comparison with the loading control GAPDH. (B) A bar graph showing the total traction force exerted by U2OS cells (error bars indicate SEM; n = 18, 13, 7, and 9 for WT, 1, 5, and 10 µM of Y-27632, respectively; **, P < 0.01; ***, P < 0.001 with respect to WT). (C) Immunostaining of actin, phosphorylated FAK (FAK-pY397), and Pxn in U2OS cells treated with varying concentrations of Y-27632. The bottom row represents the mean ratio of the background-subtracted FAK-pY397 to Pxn signal at each focal adhesion. Bar, 10 µm. (D) Bar graph of the mean ratio of FAK-pY397:Pxn for WT, 1, 5, and 10 µM of Y-27632 (error bars indicate SEM; _n_ > 500 focal adhesions from at least 10 cells for each condition). (E) The mean intensity (Inten.) of FAK-pY397 or Pxn plotted against the mean intensity of actin at each focal adhesion (FA) for the focal adhesions shown in the bottom row of C. A.U., arbitrary unit. (F) Immunostaining of actin, phosphorylated Pxn (Pxn-pY31), and Pxn in U2OS cells treated with Y-27632. The bottom row represents the mean ratio of the background-subtracted Pxn-pY31 to Pxn signal at each focal adhesion. Bar, 10 µm. (G) Bar graph of the mean ratio of Pxn-pY31:Pxn for WT, 1, 5, and 10 µM of Y-27632 (error bars indicate SEM; n > 500 focal adhesions from at least 10 cells for each condition). (H) The mean intensity of Pxn-pY31 or Pxn plotted against the mean intensity of actin at each focal adhesion for the focal adhesions shown in the bottom row of F. (I) Representative immunostained images of actin, Pxn, and fibronectin in 3T3s treated with varying concentrations of Y-27632. Bar, 30 µm.
Figure 8.
Distinct roles of actin architecture and tension in focal adhesion maturation. (1) Nascent adhesions under low tension (0–0.3 nN) assemble in the lamellipodia and turnover on minute time scales. (2) A small amount of tension (∼1 nN) generated by myosin II in the lamella stabilizes the adhesions to the ECM. Accumulation of F-actin at the adhesion occurs via an RSF template mediated by Dia1-driven polymerization of F-actin and stabilized by α-actinin cross-linking. (3) The RSF template promotes recruitment of focal adhesion (FA) proteins to facilitate compositional changes. (4) Lamellar retrograde flow drives elongation of the RSF template into an elongated stress fiber as well as adhesion growth. The maturation process that ultimately results in fibrillogenesis can occur over a large range of myosin-generated stresses but is quite sensitive to the actin density at the adhesion plaque. Alternately, compositionally immature adhesions (2) are sufficient to transmit large traction stresses to the ECM, with the stress magnitude dependent on the nature of force transmission in the actin cytoskeleton rather than the size or composition of the adhesion plaque.
Similar articles
- α-actinin1 and 4 tyrosine phosphorylation is critical for stress fiber establishment, maintenance and focal adhesion maturation.
Feng Y, Ngu H, Alford SK, Ward M, Yin F, Longmore GD. Feng Y, et al. Exp Cell Res. 2013 May 1;319(8):1124-35. doi: 10.1016/j.yexcr.2013.02.009. Epub 2013 Feb 27. Exp Cell Res. 2013. PMID: 23454549 Free PMC article. - Myosin II-mediated focal adhesion maturation is tension insensitive.
Stricker J, Beckham Y, Davidson MW, Gardel ML. Stricker J, et al. PLoS One. 2013 Jul 29;8(7):e70652. doi: 10.1371/journal.pone.0070652. Print 2013. PLoS One. 2013. PMID: 23923013 Free PMC article. - Transient frictional slip between integrin and the ECM in focal adhesions under myosin II tension.
Aratyn-Schaus Y, Gardel ML. Aratyn-Schaus Y, et al. Curr Biol. 2010 Jul 13;20(13):1145-53. doi: 10.1016/j.cub.2010.05.049. Epub 2010 Jun 10. Curr Biol. 2010. PMID: 20541412 Free PMC article. - Focal adhesions, stress fibers and mechanical tension.
Burridge K, Guilluy C. Burridge K, et al. Exp Cell Res. 2016 Apr 10;343(1):14-20. doi: 10.1016/j.yexcr.2015.10.029. Epub 2015 Oct 28. Exp Cell Res. 2016. PMID: 26519907 Free PMC article. Review. - Assembly and mechanosensory function of focal adhesions: experiments and models.
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Bershadsky AD, et al. Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review.
Cited by
- Defective CFTR modulates mechanosensitive channels TRPV4 and PIEZO1 and drives endothelial barrier failure.
Amoakon JP, Lee J, Liyanage P, Arora K, Karlstaedt A, Mylavarapu G, Amin R, Naren AP. Amoakon JP, et al. iScience. 2024 Aug 9;27(9):110703. doi: 10.1016/j.isci.2024.110703. eCollection 2024 Sep 20. iScience. 2024. PMID: 39252977 Free PMC article. - Collagen, stiffness, and adhesion: the evolutionary basis of vertebrate mechanobiology.
Tang VW. Tang VW. Mol Biol Cell. 2020 Aug 1;31(17):1823-1834. doi: 10.1091/mbc.E19-12-0709. Mol Biol Cell. 2020. PMID: 32730166 Free PMC article. Review. - Differential effect of actomyosin relaxation on the dynamic properties of focal adhesion proteins.
Lavelin I, Wolfenson H, Patla I, Henis YI, Medalia O, Volberg T, Livne A, Kam Z, Geiger B. Lavelin I, et al. PLoS One. 2013 Sep 9;8(9):e73549. doi: 10.1371/journal.pone.0073549. eCollection 2013. PLoS One. 2013. PMID: 24039980 Free PMC article. - Biophysical Approaches for Applying and Measuring Biological Forces.
Sun W, Gao X, Lei H, Wang W, Cao Y. Sun W, et al. Adv Sci (Weinh). 2022 Feb;9(5):e2105254. doi: 10.1002/advs.202105254. Epub 2021 Dec 19. Adv Sci (Weinh). 2022. PMID: 34923777 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials