Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro - PubMed (original) (raw)
Comparative Study
Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro
Anand G Patel et al. Clin Cancer Res. 2012.
Abstract
Purpose: Poly(ADP-ribose) polymerase (PARP) inhibitors are undergoing extensive clinical testing for their single-agent activity in homologous recombination (HR)-deficient tumors and ability to enhance the action of certain DNA-damaging agents. Compared with other PARP inhibitors in development, iniparib (4-iodo-3-nitrobenzamide) is notable for its simple structure and the reported ability of its intracellular metabolite 4-iodo-3-nitrosobenzamide to covalently inhibit PARP1 under cell-free conditions. The present preclinical studies were conducted to compare the actions iniparib with the more extensively characterized PARP inhibitors olaparib and veliparib.
Experimental design: The abilities of iniparib, olaparib, and veliparib to (i) selectively induce apoptosis or inhibit colony formation in HR-deficient cell lines, (ii) selectively sensitize HR-proficient cells to topoisomerase I poisons, and (iii) inhibit formation of poly(ADP-ribose) polymer (pADPr) in intact cells were compared.
Results: Consistent with earlier reports, olaparib and veliparib selectively induced apoptosis and inhibited colony formation in cells lacking BRCA2 or ATM. Moreover, like earlier generation PARP inhibitors, olaparib and veliparib sensitized cells to the topoisomerase I poisons camptothecin and topotecan. Finally, olaparib and veliparib inhibited formation of pADPr in intact cells. In contrast, iniparib exhibited little or no ability to selectively kill HR-deficient cells, sensitize cells to topoisomerase I poisons, or inhibit pADPr formation in situ. In further experiments, iniparib also failed to sensitize cells to cisplatin, gemcitabine, or paclitaxel.
Conclusions: While iniparib kills normal and neoplastic cells at high (>40 μmol/L) concentrations, its effects are unlikely to reflect PARP inhibition and should not be used to guide decisions about other PARP inhibitors.
Conflict of interest statement
Conflicts of interest: None
Figures
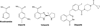
Figure 1. Structures of nicotinamide and the three drugs used in this study
Figure 2. Effects of veliparib, olaparib and iniparib on PEO1 and PEO4 cells
A, PEO1 and PEO4 treated with diluent, 20 µM veliparib (left), 5 µM olaparib (middle) or 80 µM iniparib (right) were stained with propidium iodide (PI) and subjected to flow microfluorimetry. B, summary of results obtained in panel A and additional concentrations. Inset in B, whole cell lysates were subjected to immunoblotting with antibody to BRCA2 and, as a loading control, PARP1. C, effects of various concentrations of veliparib (left), olaparib (middle) and iniparib (right) on colony formation. Error bars in these and subsequent colony forming assays, ± SD of triplicate aliquots. All results are representative of ≥ 3 independent experiments.
Figure 3. Effects of veliparib, olaparib and iniparib on paired GM16666 (ATM-deficient) and GM16667 (ATM restored) human fibroblasts
A, cells treated with diluent, 20 µM veliparib (left), 5 µM olaparib (middle) or 80 µM iniparib (right) were stained with PI and examined by flow microfluorimetry as in Fig. 2A. B, effects of veliparib (left), olaparib (middle) and iniparib (right) on colony formation. Inset in B, whole cell lysates were subjected to immunoblotting for ATM and, as a loading control, heat shock protein 90β (HSP90β). C, Atm−/− and wildtype MEFs examined by colony forming assays.
Figure 4. Inability of iniparib to sensitize cells to topoisomerase I poisons
MEFs (A, B) or SKOV3 cells (C) were treated continuously with the indicated concentrations of camptothecin (A, B) or topotecan (C) in the presence of 100 nM veliparib, 100 nM olaparib or 10 µM iniparib, then examined for colony formation.
Figure 5. Effects of iniparib in combination with cisplatin, gemcitabine, paclitaxel or etoposide
SKOV3 cells were treated continuously with the indicated concentrations of cisplatin (A), gemcitabine (C), paclitaxel (E) or etoposide (F) in the presence of diluent or 10 µM iniparib and examined for colony formation. In panels B and D, the ATR inhibitor VE-821 (1 µM), which is known to sensitize other cells to cisplatin (34), or the Chk1 inhibitor AZD 7762 (100 nM), which is known to sensitize to gemcitabine (35), was substituted for imiparib as a positive control. Error bars in each panel, ± SD of triplicate samples.
Figure 6. Effects of veliparib, olaparib and iniparib on pADPr synthesis
A, following a 4-h treatment with the indicated agent, SKOV3 cells were treated with MMS in the continued presence of the small molecule inhibitor, fixed, stained for pADPr and examined by confocal microscopy. B, mean fluorescence of pADPr in nuclei after treatment of cells with diluent (open bar) or 1 mM MMS (closed bars) in the presence of diluent or the indicated agent at 1, 10 and 100 µM. Error bars, ± SEM for 30 nuclei.
Similar articles
- Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes.
Patel AG, Flatten KS, Schneider PA, Dai NT, McDonald JS, Poirier GG, Kaufmann SH. Patel AG, et al. J Biol Chem. 2012 Feb 3;287(6):4198-210. doi: 10.1074/jbc.M111.296475. Epub 2011 Dec 12. J Biol Chem. 2012. PMID: 22158865 Free PMC article. - Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor.
Liu X, Shi Y, Maag DX, Palma JP, Patterson MJ, Ellis PA, Surber BW, Ready DB, Soni NB, Ladror US, Xu AJ, Iyer R, Harlan JE, Solomon LR, Donawho CK, Penning TD, Johnson EF, Shoemaker AR. Liu X, et al. Clin Cancer Res. 2012 Jan 15;18(2):510-23. doi: 10.1158/1078-0432.CCR-11-1973. Epub 2011 Nov 29. Clin Cancer Res. 2012. PMID: 22128301 - Comparative antiproliferative effects of iniparib and olaparib on a panel of triple-negative and non-triple-negative breast cancer cell lines.
Pierce A, McGowan PM, Cotter M, Mullooly M, O'Donovan N, Rani S, O'Driscoll L, Crown J, Duffy MJ. Pierce A, et al. Cancer Biol Ther. 2013 Jun;14(6):537-45. doi: 10.4161/cbt.24349. Cancer Biol Ther. 2013. PMID: 23760496 Free PMC article. - Poly(ADP-ribose) polymerase inhibition: a new direction for BRCA and triple-negative breast cancer?
Plummer R. Plummer R. Breast Cancer Res. 2011 Aug 16;13(4):218. doi: 10.1186/bcr2877. Breast Cancer Res. 2011. PMID: 21884642 Free PMC article. Review.
Cited by
- Poly(ADP-Ribose) Polymerase (PARP) Inhibitors for Cancer Therapy: Advances, Challenges, and Future Directions.
Bondar D, Karpichev Y. Bondar D, et al. Biomolecules. 2024 Oct 9;14(10):1269. doi: 10.3390/biom14101269. Biomolecules. 2024. PMID: 39456202 Free PMC article. Review. - HER2 overexpression renders human breast cancers sensitive to PARP inhibition independently of any defect in homologous recombination DNA repair.
Nowsheen S, Cooper T, Bonner JA, LoBuglio AF, Yang ES. Nowsheen S, et al. Cancer Res. 2012 Sep 15;72(18):4796-806. doi: 10.1158/0008-5472.CAN-12-1287. Cancer Res. 2012. PMID: 22987487 Free PMC article. - PARP-mediated repair, homologous recombination, and back-up non-homologous end joining-like repair of single-strand nicks.
Metzger MJ, Stoddard BL, Monnat RJ Jr. Metzger MJ, et al. DNA Repair (Amst). 2013 Jul;12(7):529-34. doi: 10.1016/j.dnarep.2013.04.004. Epub 2013 May 16. DNA Repair (Amst). 2013. PMID: 23684799 Free PMC article. - Investigational agents in development for the treatment of ovarian cancer.
Westin SN, Herzog TJ, Coleman RL. Westin SN, et al. Invest New Drugs. 2013 Feb;31(1):213-29. doi: 10.1007/s10637-012-9837-3. Epub 2012 Jun 4. Invest New Drugs. 2013. PMID: 22661305 Free PMC article. Review. - Using multiple targeted therapies in oncology: considerations for use, and progress to date in breast cancer.
Kelly CM, Buzdar AU. Kelly CM, et al. Drugs. 2013 May;73(6):505-15. doi: 10.1007/s40265-013-0044-0. Drugs. 2013. PMID: 23605692 Review.
References
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. - PubMed
- Rehman FL, Lord CJ, Ashworth A. Synthetic lethal approaches to breast cancer therapy. Nat Rev Clin Oncol. 2010;7:718–724. - PubMed
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. - PubMed
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. - PubMed
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA136393/CA/NCI NIH HHS/United States
- P50 CA136393-04/CA/NCI NIH HHS/United States
- T32 GM072474/GM/NIGMS NIH HHS/United States
- T32 GM072474-07/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous