Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers - PubMed (original) (raw)
Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers
Nicola J Lomas et al. Int J Clin Exp Pathol. 2012.
Abstract
Aim: This study explored the cellular and biological interrelationships involved in Idiopathic Pulmonary Fibrosis (IPF) lung tissue remodelling using immunohistochemical analysis.
Methods and results: IPF and control lung tissues were examined for localisation of Epithelial Mesenchymal Transition (EMT), proliferation and growth factor markers assessing their relationship to key histological aberrations. E-cadherin was expressed in IPF and control (Alveolar type II) ATII cells (>75%). In IPF, mean expression of N-cadherin was scanty (<10%): however 4 cases demonstrated augmented expression in ATII cells correlating to histological disease status (Pearson correlation score 0.557). Twist was expressed within fibroblastic foci but not in ATII cells. Transforming Growth Factor- β (TGF-β) protein expression was significantly increased in IPF ATII cells with variable expression within fibroblastic foci. Antigen Ki-67 was observed within hyperplastic ATII cells but not in cells overlying foci. Collagen I and α-smooth muscle actin (α-SMA) were strongly expressed within fibroblastic foci (>75%); cytoplasmic collagen I in ATII cells was present in 3 IPF cases. IPF ATII cells demonstrated variable Surfactant Protein-C (SP-C).
Conclusions: The pathogenesis of IPF is complex and involves multiple factors, possibly including EMT. Histological analysis suggests TGF-β-stimulated myofib rob lasts initiate a contractile response within established fibroblastic foci while proliferating ATII cells attempt to instigate alveolar epithelium repair. Marker expression (N-cadherin and Ki-67) correlation with histological disease activity (as reflected by fibroblastic foci extent) may emerge as future prognostic indicators for IPF.
Keywords: Idiopathic Pulmonary Fibrosis; epithelial-mesenchymal transition; immunohistochemistry; prognostic markers; tissue repair and remodelling.
Figures
Figure 1
Representative histological images of IPF patient lung tissue stained with H&E. A. IPF lung tissue showing a high number of fibroblastic foci (score 6). B. IPF lung tissue very few fibroblastic foci (score 1). Fibroblastic foci are indicated with black arrows, magnification x100.
Figure 2
Immunohistochemical analysis of IPF and control lungs for SP-C expression. A. Patchy expression of SP-C is observed in an area of ATII hyperpla-sia in IPF lung tissue. Black arrow SP-C expression, red arrow no SP-C expression. B. Representative histological image of SP-C expression in the cytoplasm of ATII cells in control lung tissue (arrow). C. Histological image of cytoplasmic SP-C expression in hyperplastic ATII cells in IPF lung tissue. Magnification x400.
Figure 3
Representative immunohistochemical images of E-cadherin expression in IPF and control lungs. A. Histological image of E-cadherin expressed around ATII cells in control lung tissue. B. Membrane expression of E-cadherin is seen in ATII cells overlying areas of fibroblastic foci in IPF lung tissue. Counterstained Alcian Blue with nuclear fast red. Magnification x200.
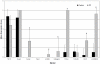
Figure 10
Semi-quantitative analysis of target molecule expression within ATII cells of IPF and control lung samples. Data are presented as mean expression score ±SD. * = significant difference in expression between the control and IPF group p≤0.05.
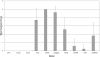
Figure 11
Semi-quantitative analysis of target molecule expression within the fibroblasticfoci of IPF lung tissue samples. Data are presented as mean expression score ±SD.
Figure 4
Dual-labelled immunohistochemistry of IPF and control lung samples for N-cadherin and Twist expression. A. Representative histological image of control lung tissue shows no N-cadherin and Twist. B. IPF lung tissue demonstrates Twist reactivity within the fibroblastic foci (arrow). Magnification A x100, B x400.
Figure 5
Immunohistochemical analysis of IPF and control lungs for the mesenchymal marker α-SMA expression. A. Representative histological image of α-SMA expression around a blood vessel in control lung. B. α-SMA expression within fibroblastic foci in IPF lung tissue demonstrates the population of cells being composed of myofibroblasts. Magnification panel A x200, panel B x400.
Figure 6
Immunohistochemical analysis of IPF and control lungs for collagen I expression. A. Representative histological image of collagen I immunoreactivity surrounding a vessel in control lung tissue. B. Collagen I immunoreactivity within a fibroblasticfoci is seen in IPF lung tissue. Magnification x200.
Figure 7
Immunohistochemical analysis of IPF and control lung samples labelled for either TGF- β protein or TGF- β receptor. A. Representative histological image showing no cytoplasmic TGF-β protein immunoreactivity in control lung tissue. B. Cytoplasmic TGF-β protein is seen in ATII cells overlyingfibroblastic foci in a representative histological image of IPF lung tissue. C. TGF-β receptor immunoreactivity is not observed in control lung tissue. D. TGF-β receptor immunoreactivity can be seen in lymphocytes in IPF lung tissue. Magnification A-C x200, D x400.
Figure 8
Immunohistochemical analysis of IPF and control lungs for the cell proliferation marker antigen Ki-67. A. Representative histological image shows antigen Ki-67 is not observed in control lung tissue. B. A representative histological image of IPF lung tissue shows antigen Ki-67 immunoreactivity is observed in the nucleus of ATII cells at the edges of fibroblastic foci (arrow) but not in the ATII cells directly overlying the area. Magnification x200.
Figure 9
Immunohistochemical analysis of IPF and control lungs for p16INK4A . A. p16INK4A reactivity is not observed in control lung tissue in this representative histological image. B. Cytoplasmic p16INK4A reactivity is seen in the ATII cells overlying fibroblastic foci (arrow) in a representative histological image of IPF lung tissue. Magnification x400.
Similar articles
- Bidirectional epithelial-mesenchymal crosstalk provides self-sustaining profibrotic signals in pulmonary fibrosis.
Yao L, Zhou Y, Li J, Wickens L, Conforti F, Rattu A, Ibrahim FM, Alzetani A, Marshall BG, Fletcher SV, Hancock D, Wallis T, Downward J, Ewing RM, Richeldi L, Skipp P, Davies DE, Jones MG, Wang Y. Yao L, et al. J Biol Chem. 2021 Sep;297(3):101096. doi: 10.1016/j.jbc.2021.101096. Epub 2021 Aug 18. J Biol Chem. 2021. PMID: 34418430 Free PMC article. - Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition.
Milara J, Navarro R, Juan G, Peiró T, Serrano A, Ramón M, Morcillo E, Cortijo J. Milara J, et al. Thorax. 2012 Feb;67(2):147-56. doi: 10.1136/thoraxjnl-2011-200026. Epub 2011 Nov 21. Thorax. 2012. PMID: 22106015 - The Oncogene ECT2 Contributes to a Hyperplastic, Proliferative Lung Epithelial Cell Phenotype in Idiopathic Pulmonary Fibrosis.
Ulke HM, Mutze K, Lehmann M, Wagner DE, Heinzelmann K, Günther A, Eickelberg O, Königshoff M. Ulke HM, et al. Am J Respir Cell Mol Biol. 2019 Dec;61(6):713-726. doi: 10.1165/rcmb.2019-0047OC. Am J Respir Cell Mol Biol. 2019. PMID: 31145635 - Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF).
Confalonieri P, Volpe MC, Jacob J, Maiocchi S, Salton F, Ruaro B, Confalonieri M, Braga L. Confalonieri P, et al. Cells. 2022 Jun 30;11(13):2095. doi: 10.3390/cells11132095. Cells. 2022. PMID: 35805179 Free PMC article. Review. - Epithelial-to-mesenchymal transition and its role in EGFR-mutant lung adenocarcinoma and idiopathic pulmonary fibrosis.
Sakuma Y. Sakuma Y. Pathol Int. 2017 Aug;67(8):379-388. doi: 10.1111/pin.12553. Epub 2017 Jul 5. Pathol Int. 2017. PMID: 28678431 Review.
Cited by
- WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis.
Meuten T, Hickey A, Franklin K, Grossi B, Tobias J, Newman DR, Jennings SH, Correa M, Sannes PL. Meuten T, et al. Respir Res. 2012 Jul 28;13(1):62. doi: 10.1186/1465-9921-13-62. Respir Res. 2012. PMID: 22838404 Free PMC article. - Senescent AECⅡ and the implication for idiopathic pulmonary fibrosis treatment.
Zhang T, Zhang J, Lv C, Li H, Song X. Zhang T, et al. Front Pharmacol. 2022 Nov 15;13:1059434. doi: 10.3389/fphar.2022.1059434. eCollection 2022. Front Pharmacol. 2022. PMID: 36457712 Free PMC article. Review. - Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis.
Song X, Cao G, Jing L, Lin S, Wang X, Zhang J, Wang M, Liu W, Lv C. Song X, et al. J Cell Mol Med. 2014 Jun;18(6):991-1003. doi: 10.1111/jcmm.12243. Epub 2014 Apr 6. J Cell Mol Med. 2014. PMID: 24702795 Free PMC article. - Melittin Exerts Beneficial Effects on Paraquat-Induced Lung Injuries In Mice by Modifying Oxidative Stress and Apoptosis.
El-Aarag B, Magdy M, AlAjmi MF, Khalifa SAM, El-Seedi HR. El-Aarag B, et al. Molecules. 2019 Apr 16;24(8):1498. doi: 10.3390/molecules24081498. Molecules. 2019. PMID: 30995821 Free PMC article. - Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches.
Akram KM, Patel N, Spiteri MA, Forsyth NR. Akram KM, et al. Int J Mol Sci. 2016 Jan 19;17(1):128. doi: 10.3390/ijms17010128. Int J Mol Sci. 2016. PMID: 26797607 Free PMC article. Review.
References
- Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in IPF. Am J Respir Crit Care Med. 2002;166:173–177. 15. - PubMed
- King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniak RM. Am J Respir Crit Care Med. 2001;164(6):1025–1032. - PubMed
- Barlo NP, van Moorsel CH, van den Bosch JM, Grutters JC. Predicting prognosis in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):85–95. - PubMed
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and mechanisms? Eur Respir J. 2007;30(5):835–839. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials