Accessory molecules for Toll-like receptors and their function - PubMed (original) (raw)
Review
Accessory molecules for Toll-like receptors and their function
Clarissa C Lee et al. Nat Rev Immunol. 2012.
Abstract
Toll-like receptors (TLRs) are essential components of the innate immune system. Accessory proteins are required for the biosynthesis and activation of TLRs. Here, we summarize recent findings on TLR accessory proteins that are required for cell-surface and endosomal TLR function, and we classify these proteins based on their function as ligand-recognition and delivery cofactors, chaperones and trafficking proteins. Because of their essential roles in TLR function, targeting of such accessory proteins may benefit strategies aimed at manipulating TLR activation for therapeutic applications.
Figures
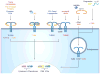
Figure 1. Accessory molecules mediate ligand binding and delivery to surface and endosomal TLRs
The TLR1-TLR2 heterodimer utilizes CD14 to respond to triacyl lipopetides. The TLR2-TLR6 heterodimer uses CD14 to respond to zymosan and both CD14 and CD36 to respond to lipoteichoic acid (LTA) and diacyl lipopeptides. LPS-binding protein (LBP) binds LPS and presents it to CD14, which is required for TRIF-dependent signaling in response to LPS and at low doses for MyD88-dependent signaling. TLR4 requires MD2 to bind LPS and homodimerize. CD36 is required by the TLR4-TLR6 heterodimer to respond to the altered self-components, amyloid-β and oxidized LDL (oxLDL). Endosomal TLRs utilize cofactors for nucleic acid delivery. CD14 and HMGBs bind to dsRNA, ssRNA, and DNA and mediate their delivery to TLR3, TLR7, and TLR9, respectively. LL37 binds both RNA and DNA and delivers it to TLR7, TLR8 (RNA) and TLR9 (DNA). Progranulin bind just to DNA, and mediate DNA delivery to TLR9. Signaling from TLRs culminates in the activation of the transcription factors AP1, NF-κB, and interferon-regulatory factors (IRFs) and the production of pro-inflammatory cytokines and type I interferons (not shown).
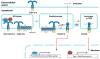
Figure 2. ER chaperones and trafficking and processing factors of TLRs
The ER luminal chaperones gp96 and PRAT4A are responsible for proper folding and function of TLR1, TLR2, TLR4, TLR7 and TLR9, but not TLR3. The ER membrane protein UNC93B1 is required for translocation of endosomal TLR7 and TLR9 to endolysosomes, where these TLRs are cleaved by cathepsins and asparagine endopeptidase (AEP). The cleaved TLRs bind ligand (DNA or RNA) triggering recruitment of signaling components leading to NF-κB-dependent proinflammatory cytokine production. The adaptor protein 3 (AP3) mediates translocation of TLR9 to LAMP2+ lysosome or lysosome-related organelles (LRO), where the IRF7 signaling pathway is initiated leading to the expression of type I interferon genes.
Similar articles
- [Toll-like receptors: recognition receptors of the innate immune system and target structures for therapeutical intervention].
Kabelitz D. Kabelitz D. Med Monatsschr Pharm. 2012 Jul;35(7):238-44. Med Monatsschr Pharm. 2012. PMID: 22852274 Review. German. - RAGE and TLRs: relatives, friends or neighbours?
Ibrahim ZA, Armour CL, Phipps S, Sukkar MB. Ibrahim ZA, et al. Mol Immunol. 2013 Dec;56(4):739-44. doi: 10.1016/j.molimm.2013.07.008. Epub 2013 Aug 14. Mol Immunol. 2013. PMID: 23954397 Review. - Toll-like receptors and Type I interferons.
Uematsu S, Akira S. Uematsu S, et al. J Biol Chem. 2007 May 25;282(21):15319-23. doi: 10.1074/jbc.R700009200. Epub 2007 Mar 29. J Biol Chem. 2007. PMID: 17395581 Review. - Cell Surface Expression of Endosomal Toll-Like Receptors-A Necessity or a Superfluous Duplication?
Mielcarska MB, Bossowska-Nowicka M, Toka FN. Mielcarska MB, et al. Front Immunol. 2021 Feb 1;11:620972. doi: 10.3389/fimmu.2020.620972. eCollection 2020. Front Immunol. 2021. PMID: 33597952 Free PMC article. Review. - Retinal photoreceptor expresses toll-like receptors (TLRs) and elicits innate responses following TLR ligand and bacterial challenge.
Singh PK, Kumar A. Singh PK, et al. PLoS One. 2015 Mar 13;10(3):e0119541. doi: 10.1371/journal.pone.0119541. eCollection 2015. PLoS One. 2015. PMID: 25767877 Free PMC article.
Cited by
- Short Link N Modulates Inflammasome Activity in Intervertebral Discs Through Interaction with CD14.
Alad M, Grant MP, Epure LM, Shih SY, Merle G, Im HJ, Antoniou J, Mwale F. Alad M, et al. Biomolecules. 2024 Oct 16;14(10):1312. doi: 10.3390/biom14101312. Biomolecules. 2024. PMID: 39456246 Free PMC article. - Immunological perspectives on atherosclerotic plaque formation and progression.
Pi H, Wang G, Wang Y, Zhang M, He Q, Zheng X, Yin K, Zhao G, Jiang T. Pi H, et al. Front Immunol. 2024 Sep 27;15:1437821. doi: 10.3389/fimmu.2024.1437821. eCollection 2024. Front Immunol. 2024. PMID: 39399488 Free PMC article. Review. - The Battle of LPS Clearance in Host Defense vs. Inflammatory Signaling.
Kumar P, Schroder EA, Rajaram MVS, Harris EN, Ganesan LP. Kumar P, et al. Cells. 2024 Sep 21;13(18):1590. doi: 10.3390/cells13181590. Cells. 2024. PMID: 39329771 Free PMC article. Review. - The role of TIR domain-containing proteins in bacterial defense against phages.
Wang S, Kuang S, Song H, Sun E, Li M, Liu Y, Xia Z, Zhang X, Wang X, Han J, Rao VB, Zou T, Tan C, Tao P. Wang S, et al. Nat Commun. 2024 Aug 27;15(1):7384. doi: 10.1038/s41467-024-51738-3. Nat Commun. 2024. PMID: 39191765 Free PMC article. - Phospholipid peroxidation in macrophage confers tumor resistance by suppressing phagocytic capability towards ferroptotic cells.
Luo X, Gong HB, Li ZC, Li DD, Li ZX, Sun J, Yan CY, Huang RT, Feng Y, Chen SR, Cao YF, Liu M, Wang R, Huang F, Sun WY, Kurihara H, Duan WJ, Liang L, Jin W, Wu YP, He RR, Li YF. Luo X, et al. Cell Death Differ. 2024 Sep;31(9):1184-1201. doi: 10.1038/s41418-024-01351-0. Epub 2024 Aug 5. Cell Death Differ. 2024. PMID: 39103535
References
- Janeway CA, Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. - PubMed
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. - PubMed
- Kang JY, Lee JO. Structural biology of the toll-like receptor family. Annu Rev Biochem. 2011;80:917–941. - PubMed
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. - PubMed
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources