Direct role of Bardet-Biedl syndrome proteins in transcriptional regulation - PubMed (original) (raw)
. 2012 Jan 15;125(Pt 2):362-75.
doi: 10.1242/jcs.089375. Epub 2012 Feb 2.
Affiliations
- PMID: 22302990
- PMCID: PMC3283873
- DOI: 10.1242/jcs.089375
Direct role of Bardet-Biedl syndrome proteins in transcriptional regulation
Cecilia Gascue et al. J Cell Sci. 2012.
Abstract
Primary cilia are conserved organelles that play crucial roles as mechano- and chemosensors, as well as transducing signaling cascades. Consequently, ciliary dysfunction results in a broad range of phenotypes: the ciliopathies. Bardet-Biedl syndrome (BBS), a model ciliopathy, is caused by mutations in 16 known genes. However, the biochemical functions of the BBS proteins are not fully understood. Here we show that the BBS7 protein (localized in the centrosomes, basal bodies and cilia) probably has a nuclear role by virtue of the presence of a biologically confirmed nuclear export signal. Consistent with this observation, we show that BBS7 interacts physically with the polycomb group (PcG) member RNF2 and regulate its protein levels, probably through a proteasome-mediated mechanism. In addition, our data supports a similar role for other BBS proteins. Importantly, the interaction with this PcG member is biologically relevant because loss of BBS proteins leads to the aberrant expression of endogenous RNF2 targets in vivo, including several genes that are crucial for development and for cellular and tissue homeostasis. Our data indicate a hitherto unappreciated, direct role for the BBS proteins in transcriptional regulation and potentially expand the mechanistic spectrum that underpins the development of ciliary phenotypes in patients.
Figures
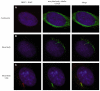
Fig. 1.
BBS7 has a dynamic localization pattern. (A–C) Immunocytochemistry assays in NIH3T3 cells using antibodies against endogenous BBS7 (red, left column) and γ- and acetylated tubulin to label centrosomes, basal bodies and the ciliary axoneme (green, middle column). BBS7 localizes to the pericentriolar region (A), to the basal body (B), and to the basal body and the primary cilium (C). DNA was stained with DAPI (blue).
Fig. 2.
BBS7 can enter the nuclear compartment. (A) Immunofluorescence staining of NIH3T3 with a high concentration of the BBS7 antibody in untreated cells (upper panel) and in cells treated with LMB (bottom panel). In both cases, BBS7 staining is shown in green in the left column, the merge with γ- and acetylated tubulin and DAPI is shown in the right column. (B) Immunofluorescence staining of NIH3T3 cells showing the formation of nuclear aggregates that are evident at normal concentrations of BBS7 antibody after treatment with NEM (red). The nucleus is stained with DAPI (blue) and the γ- and acetylated tubulin are shown in green. (C) Cell fractionation showing the presence of endogenous BBS7 in both cytoplasmic and nuclear compartments; antibodies against γ-tubulin and histone H3 were used as controls for each fraction. (D) Epifluorescence images of NIH3T3 cells expressing Myc–BBS7 wild-type (upper panel) and Myc–BBS7 NES (bottom panel) using an anti-Myc antibody (green). The graph shows the percentage of cells transfected with Myc–BBS7 wild-type, Myc–BBS7 NES and the BBS7 mutants H323R and T211I, showing either a cytoplasmic distribution with nuclear exclusion (categorized as cytoplasmic) versus cytoplasmic and nuclear staining. Values shown means ± s.d. (E) Cell fractionation after transfection with Myc–EV, Myc–BBS7 wild-type and Myc–BBS7 NES showing that Myc–BBS7 NES is enriched in the nucleus; antibodies against γ-tubulin and histone H3 were used as controls.
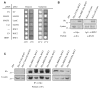
Fig. 3.
BBS proteins interact with RNF2. (A) Cytoplasmic yeast two-hybrid screen using BBS7 as bait resulted in the identification of RNF2. True positives, MAFB-MAFB (positive control) and BBS7-RNF2 are shown growing at the restrictive temperature of 37°C only in galactose (condition in which the prey is expressed). Negative controls include MAFB-Lamin C, Coll-MAFB and both empty vectors, EV-EV. (B) Co-immunoprecipitation assays showing that BBS7 interacts with RNF2 in mammalian cells. Left: Cell lysates of HEK293 cells transiently expressing constructs (as indicated) were used to immunoprecipitate with an anti-Myc antibody. Both immunoprecipitates and cell lysates were analyzed by SDS-PAGE with an anti-HA antibody. Right: Endogenous BBS7 interacts with endogenous RNF2. Lysates of untreated HeLa cells were immunoprecipitated with an anti-BBS7 antibody and purified rabbit IgGs as control, and western-blotted with a mouse monoclonal anti-RNF2 antibody. (C) BBS1, BBS2, BBS4, BBS5, BBS6, BBS8 and BBS10, but not an unrelated protein (Myc–Control), can also interact with RNF2 in mammalian cells.
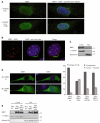
Fig. 4.
Depletion of BBS proteins affects proteasome efficiency and RNF2 protein levels. (A) Western blots of cell extracts after transient inhibition of BBS7 and RNF2 with pSUPER vectors (using the empty vector, pSUPER EV, as control) and blotted with an anti-RNF2 antibody (upper panel) and an anti-BBS7 (lower panel). An anti-GAPDH antibody was used as a loading control. (B) RNF2 average band density quantification of the western blot (considering the three biological replicates), including the effect of the inhibition of BBS1, BBS2 and BBS4 (blots not shown). (C) Immunofluorescence image of HEK293 proteasome sensor line cells transfected with empty vector (left) and pSUPER BBS7 (right), showing the increased GFP signal in BBS7-depleted cells. (D) Quantification of the GFP signal measured by flow cytometry and expressed as fold change. Values shown means ± s.d.
Fig. 5.
BBS7 depletion alters the expression of RNF2 target genes. (A,B) Relative expression levels of RNF2 and 11 previously identified RNF2 target genes analyzed by semi-quantitative (A) and Real Time RT-PCR (B) in BBS7-depleted cells (pSUPER BBS7) relative to control cells (pSUPER EV). GAPDH and BBS7 were amplified as controls. The relative expression of each gene is shown as the fold change and the error bars represent the s.d. of replicates. The cross-bars in the _y_-axis indicate changes in the scale used.
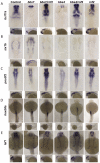
Fig. 6.
In vivo misregulation of RNF2 target genes in zebrafish BBS morphants. (A–E) Embryos injected with bbs7, bbs4 or rnf2 MOs and stained by in situ hybridization at 30 hours post-fertilization using probes for bcl11a, six1b, pou3f2, hoxb8a and lef1. (A) Embryos injected with bbs7 and bbs4 MOs had significantly less staining of bcl11a than uninjected control embryos whereas co-injection of bbs7/4 with rnf2 rescued and even expanded the field of expression. (B) six1b show an altered pattern of expression with staining in the diencephalon in bbs morphants. (C) pou3f2 staining showed lower levels of expression in bbs7 and bbs4 morphants, with rescue of expression in double morphants of bbs7/rnf2 and bbs4/rnf2. (D) hoxb8a expression in bbs7, bbs4 and double morphants with rnf2 showed no significant difference in expression. (E) lef1 expression is reduced in morphant embryos.
Fig. 7.
Microarray analysis of BBS7-depleted cells. Transiently transfected HeLa cells were used to compare the transcriptional profile of control and BBS7-depleted cells. (A) Boxplot of the log _P_-values obtained in the microarray experiment for the genes included in the cilia proteome compared with the genes in the array that are not present in that database (NO). (B) Similarly, target genes for different PcG proteins (SUZ12, EED, PHC1 and RNF2) and for genes with the trimethylated H3K27 marker (H3K27me3) compared with the genes not present in those groups (NO) and also compared with the CCND1 target genes used as a negative control (Ctrl-neg). (C) Upregulated and downregulated genes (showing P<1×10−5) in the absence of BBS7 were used in an ontology analysis. Ontology classes with an over-representation in our dataset are shown.
Similar articles
- Functional characterization of Prickle2 and BBS7 identify overlapping phenotypes yet distinct mechanisms.
Mei X, Westfall TA, Zhang Q, Sheffield VC, Bassuk AG, Slusarski DC. Mei X, et al. Dev Biol. 2014 Aug 15;392(2):245-55. doi: 10.1016/j.ydbio.2014.05.020. Epub 2014 Jun 2. Dev Biol. 2014. PMID: 24938409 Free PMC article. - Molecular architecture of the Bardet-Biedl syndrome protein 2-7-9 subcomplex.
Ludlam WG, Aoba T, Cuéllar J, Bueno-Carrasco MT, Makaju A, Moody JD, Franklin S, Valpuesta JM, Willardson BM. Ludlam WG, et al. J Biol Chem. 2019 Nov 1;294(44):16385-16399. doi: 10.1074/jbc.RA119.010150. Epub 2019 Sep 17. J Biol Chem. 2019. PMID: 31530639 Free PMC article. - Tissue-dependent differences in Bardet-Biedl syndrome gene expression.
Patnaik SR, Farag A, Brücker L, Volz AK, Schneider S, Kretschmer V, May-Simera HL. Patnaik SR, et al. Biol Cell. 2020 Feb;112(2):39-52. doi: 10.1111/boc.201900077. Epub 2020 Jan 13. Biol Cell. 2020. PMID: 31845361 - Ciliopathies and the Kidney: A Review.
McConnachie DJ, Stow JL, Mallett AJ. McConnachie DJ, et al. Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review. - Bardet-Biedl syndrome: Is it only cilia dysfunction?
Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Novas R, et al. FEBS Lett. 2015 Nov 14;589(22):3479-91. doi: 10.1016/j.febslet.2015.07.031. Epub 2015 Jul 29. FEBS Lett. 2015. PMID: 26231314 Review.
Cited by
- Bardet-Biedl syndrome: The pleiotropic role of the chaperonin-like BBS6, 10, and 12 proteins.
Gupta N, D'Acierno M, Zona E, Capasso G, Zacchia M. Gupta N, et al. Am J Med Genet C Semin Med Genet. 2022 Mar;190(1):9-19. doi: 10.1002/ajmg.c.31970. Epub 2022 Apr 4. Am J Med Genet C Semin Med Genet. 2022. PMID: 35373910 Free PMC article. Review. - Whole organism transcriptome analysis of zebrafish models of Bardet-Biedl Syndrome and Alström Syndrome provides mechanistic insight into shared and divergent phenotypes.
Hostelley TL, Lodh S, Zaghloul NA. Hostelley TL, et al. BMC Genomics. 2016 May 3;17:318. doi: 10.1186/s12864-016-2679-1. BMC Genomics. 2016. PMID: 27142762 Free PMC article. - The ciliopathies: a transitional model into systems biology of human genetic disease.
Davis EE, Katsanis N. Davis EE, et al. Curr Opin Genet Dev. 2012 Jun;22(3):290-303. doi: 10.1016/j.gde.2012.04.006. Epub 2012 May 23. Curr Opin Genet Dev. 2012. PMID: 22632799 Free PMC article. Review. - Ciliary Entry of the Hedgehog Transcriptional Activator Gli2 Is Mediated by the Nuclear Import Machinery but Differs from Nuclear Transport in Being Imp-α/β1-Independent.
Torrado B, Graña M, Badano JL, Irigoín F. Torrado B, et al. PLoS One. 2016 Aug 31;11(8):e0162033. doi: 10.1371/journal.pone.0162033. eCollection 2016. PLoS One. 2016. PMID: 27579771 Free PMC article. - Exploring Key Challenges of Understanding the Pathogenesis of Kidney Disease in Bardet-Biedl Syndrome.
Marchese E, Ruoppolo M, Perna A, Capasso G, Zacchia M. Marchese E, et al. Kidney Int Rep. 2020 Jun 29;5(9):1403-1415. doi: 10.1016/j.ekir.2020.06.017. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954066 Free PMC article. Review.
References
- Ansley S. J., Badano J. L., Blacque O. E., Hill J., Hoskins B. E., Leitch C. C., Kim J. C., Ross A. J., Eichers E. R., Teslovich T. M., et al. (2003). Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425, 628-633 - PubMed
- Badano J. L., Leitch C. C., Ansley S. J., May-Simera H., Lawson S., Lewis R. A., Beales P. L., Dietz H. C., Fisher S., Katsanis N. (2006a). Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature 439, 326-330 - PubMed
- Badano J. L., Mitsuma N., Beales P. L., Katsanis N. (2006b). The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 22, 125-148 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK072301/DK/NIDDK NIH HHS/United States
- R01 DK075972/DK/NIDDK NIH HHS/United States
- R01DK075972/DK/NIDDK NIH HHS/United States
- R01HD04260/HD/NICHD NIH HHS/United States
- R01DK072301/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases