Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections - PubMed (original) (raw)
. 2012 Feb 3;148(3):434-46.
doi: 10.1016/j.cell.2011.12.023.
Francisco J Roca, Sungwhan F Oh, Ross McFarland, Thad W Vickery, John P Ray, Dennis C Ko, Yuxia Zou, Nguyen D Bang, Tran T H Chau, Jay C Vary, Thomas R Hawn, Sarah J Dunstan, Jeremy J Farrar, Guy E Thwaites, Mary-Claire King, Charles N Serhan, Lalita Ramakrishnan
Affiliations
- PMID: 22304914
- PMCID: PMC3433720
- DOI: 10.1016/j.cell.2011.12.023
Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections
David M Tobin et al. Cell. 2012.
Abstract
Susceptibility to tuberculosis is historically ascribed to an inadequate immune response that fails to control infecting mycobacteria. In zebrafish, we find that susceptibility to Mycobacterium marinum can result from either inadequate or excessive acute inflammation. Modulation of the leukotriene A(4) hydrolase (LTA4H) locus, which controls the balance of pro- and anti-inflammatory eicosanoids, reveals two distinct molecular routes to mycobacterial susceptibility converging on dysregulated TNF levels: inadequate inflammation caused by excess lipoxins and hyperinflammation driven by excess leukotriene B(4). We identify therapies that specifically target each of these extremes. In humans, we identify a single nucleotide polymorphism in the LTA4H promoter that regulates its transcriptional activity. In tuberculous meningitis, the polymorphism is associated with inflammatory cell recruitment, patient survival and response to adjunctive anti-inflammatory therapy. Together, our findings suggest that host-directed therapies tailored to patient LTA4H genotypes may counter detrimental effects of either extreme of inflammation.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1
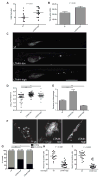
Identification of eicosanoids in zebrafish by LC-MS-MS. (A) Lipoxygenase-derived lipid mediator biosynthesis pathway from arachidonic acid and contribution of LTA4H. (B) LC-MS/MS lipid mediator lipidomics of adult zebrafish. Left: Extracted ion chromatograms of two key lipid mediators, LXA4 metabolite 13,14-dihydro-15-oxo LXA4 (351->115) and LTB4 (335->195). Right: MS/MS fragmentation and structure assignment of lipid mediators. For further MS3 analysis, see Figure S1. (C) LXA4 metabolite levels (mean ± SEM of three animals) in wildtype (wt) and lta4h mutant zebrafish P=0.02 (Student’s unpaired t-test). (D) TNF mRNA levels (mean ± SD) in pooled 3 dpi zebrafish larvae analyzed 7 hours after injection with LXA4 or its metabolite 13, 14-dihydro-15-oxo LXA4 identified using reported criteria (Serhan et al., 1993). ***, P<0.001 (one way ANOVA with Dunnett’s post-test). See also Figure S1.
Figure 2
Extremes of LTA4H expression drive hypersusceptibility in zebrafish. (A) Median number of macrophages recruited to the hindbrain ventricle of wildtype and LTA4H-high siblings 4 hrs after injection of 150-200 M. marinum into this space at 30 hpf. P=0.01 (Student’s unpaired t-test). Representative of two independent experiments. (B) TNF mRNA levels (mean ± SEM of three independent experiments) for control and LTA4H-high siblings 1 dpi with 150-200 M. marinum. P=0.02 by Student’s unpaired t-test. (C) Fluorescence images of representative wildtype, LTA4H-low and LTA4H-high larvae 3 dpi with 90-100 M. marinum. (D) Bacterial burden of all larvae from (C) by fluorescence pixel counts (FPC). **P < 0.01; ***P < 0.001. (one-way ANOVA with Tukey’s post-test; all other comparisons not significant). Representative of >7 independent experiments measuring differences in bacterial burden of the three genotypes. (E) Mean (± SEM) number of bacteria per infected macrophage in 11 wildtype larvae, 8 LTA4H-low larvae and 13 LTA4H-high larvae, 40 hpi with 150-200 erp mutant M. marinum. ***P < 0.001 (one way ANOVA with Dunnett’s post-test). Representative of two independent experiments. (F) Fluorescence images showing discrete bacterial clumps indicative of macrophage residence in wildtype versus corded extracellular bacteria in their LTA4H-low and LTA4H-high siblings at 3 dpi with 150-200 M. marinum. (G) Percentage of animals in (F) with cording among wildtype, LTA4H-low and LTA4H-high siblings 4 dpi after infection with 90-100 M. marinum. *, P<0.05; ***, P<0.001 (Fisher’s exact test comparing LTA4H-low and LTA4H-high to wildtype). (H) Quantitation of neutral red positive cells 4 dpi after infection with approximately 100 M. marinum in sibling controls and LTA4H-high animals and (I) sibling controls and LTA4H-low animals. See also Figure S2 and Movies S1-S3.
Figure 3
Model of proposed mechanism for susceptibility of LTA4H-low and –high genotypes wherein either TNF deficiency or excess results in macrophage necrosis and exuberant extracellular bacterial growth.
Figure 4
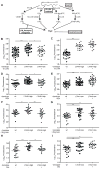
Modulation of TNF levels results in genotype-specific rescue of LTA4H-mediated hypersusceptibility. (A). Mean (± SEM) number of bacteria per infected macrophage 40-44 hpi with 150-200 erp mutant M. marinum of wildtype or TNF morphant (MO) siblings. (B) FPC in control or tnf morphant siblings 3 dpi with 90-100 M. marinum. (C) Quantitation of frequency of bacterial cording of the animals in (B). (D) Quantitation of neutral red positive cells 4 dpi after infection with 100 M. marinum in sibling controls or tnf morphants. (E) Mean (± SEM) number of bacteria per infected macrophage at 40-44 hpi in wildtype animals with or without injection of 0.5 ng rTNF 12 hours after infection with 150-200 erp mutant M. marinum. (F) FPC in control or rTNF injected siblings 3 dpi with 90-100 M. marinum. (G) Quantitation of frequency of bacterial cording of the animals in (F). (H) Quantitation of neutral red positive cells at 4 dpi after infection with 90-100 M. marinum in sibling controls or rTNF injected animals. (I) FPC in wildtype or lta4h morphant siblings 3 dpi with 90-100 M. marinum per animal after injection of 0.5 ng rTNF at 12 hpi. (J) Bacterial cording frequency of the animals in (I). (K) FPC in wildtype animals, LTA4H-high siblings, and LTA4H-high plus TNF morphant animals, at 3 dpi with 90-100 M. marinum. (L) Quantitation of frequency of bacterial cording in the animals in (K). Statistical comparisons in panels (I) and (K) by one-way ANOVA with Tukey’s post-test; in panels (C),(G),(J) and (L) by Fisher’s exact test; (A),(B),(D),(E),(F),(H) by Student’s unpaired t-test. For all panels *, P<0.05; **, P<0.01; ***, P<0.001, all other comparisons not significant. See also Figure S3.
Figure 5
Genotype-dependent therapies rescue hypersusceptibility to M. marinum in the zebrafish. (A) Pathways of LTB4 and LXA4 synthesis highlighting points of pharmacological intervention. (B) FPC of LTA4H-low or wildtype siblings in the presence or absence of 100 nM of PD146176, a 15-lipoxygenase inhibitor (15-LOXi). Representative of 2 independent experiments. (C) FPC as in (B) for LTA4H-high animals or wildtype siblings. Representative of 2 independent experiments. (D) FPC of LTA4H-high or wildtype siblings in the presence or absence of 1 μM ASA. Representative of 2 independent experiments. (E) FPC as in (D) of LTA4H-low or wildtype siblings. (F) FPC of LTA4H-high or wildtype siblings in the presence or absence of 1 μM of U75302, an antagonist of the BLT1 LTB4 receptor (LTB4-R ant). Representative of 3 independent experiments. (G) FPC as in (F) for LTA4H-low or wildtype siblings. (H) FPC of LTA4H-high and wildtype siblings in the presence or absence of 0.75 μM DEX. Representative of 3 independent experiments. (I) FPC as in (H) for LTA4H-low animals or wildtype siblings. Representative of 2 independent experiments. 150-200 M. marinum used for all infections with analyses performed at 3dpi. Statistical comparisons in all panels by one-way ANOVA with Tukey’s post-test. *, P<0.05; **, P<0.01; ***, P<0.001. See also Figures S4 and S5.
Figure 6
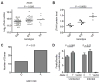
The human LTA4H promoter variant rs17525495 C/T is associated with differences in LTA4H RNA and protein expression. (A) LTA4H mRNA expression in lymphoblastoid cell lines (LCLs) from Asian individuals (CHB+JPT) (from (Stranger et al., 2007)) P=0.026 (one-way ANOVA). Data for all HapMap samples shown in Figure S6A. (B) LTA4H protein expression levels from LCLs of unrelated Han Chinese CC or TT homozygotes or CT heterozygotes, detected by Western blot and normalized to b-tubulin (also see Figure S6B). P=0.0002 (Student’s t-test). Representative of 2 independent experiments for the CC and TT homozygotes; CT heterozygotes were analyzed once in this experiment (C) Number of cDNA clones corresponding to the C or T allele at rs17525495 isolated from the heterozygous LCL GM19190. (D) Luciferase expression (mean ± SEM of 11 independent experiments) transcribed from a one kb promoter fragment immediately upstream of the LTA4H translation start site containing the rs17525495 C and T alleles. P=0.0064 for uninfected cells and P=0.02 for _M. marinum_-infected cells (paired t-test). See also Figure S6.
Figure 7
rs17525495 genotype influences TB meningitis survival, inflammation, and treatment response. (A) Mortality from TB meningitis for all patients (treated and untreated with dexamethasone (DEX)), stratified by rs17525495 genotype (P=0.02, log rank test). (B) Median pre-treatment leukocyte counts in cerebrospinal fluid stratified by rs17525495 genotype (P=0.006, one-way ANOVA). (C) Influence of adjunctive dexamethasone treatment on patient survival for all genotypes. Treatment effect is not significant (P=0.2). (D) Survival among patients not treated with dexamethasone, stratified by rs17525495 genotype (P=0.042, log rank test) (E) Survival among patients treated with dexamethasone, stratified by rs17525495 genotype (P=0.005, log rank test). See also Figure S7 and Tables S1 and S2.
Comment in
- Innate immunity to TB: a druggable balancing act.
Lalvani A, Behr MA, Sridhar S. Lalvani A, et al. Cell. 2012 Feb 3;148(3):389-91. doi: 10.1016/j.cell.2012.01.026. Cell. 2012. PMID: 22304907
References
- Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460:98–102. - PubMed
- Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. - PubMed
- Auerbach BJ, Bisgaier CL, Wolle J, Saxena U. Oxidation of low density lipoproteins greatly enhances their association with lipoprotein lipase anchored to endothelial cell matrix. J Biol Chem. 1996;271:1329–1335. - PubMed
- Behr M, Schurr E, Gros P. TB: screening for responses to a vile visitor. Cell. 2010;140:615–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- DP1 MH099901/MH/NIMH NIH HHS/United States
- DP2 OD008614/OD/NIH HHS/United States
- R37 AI054503/AI/NIAID NIH HHS/United States
- R01 AI054503/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous