Modular genetic control of sexually dimorphic behaviors - PubMed (original) (raw)
Modular genetic control of sexually dimorphic behaviors
Xiaohong Xu et al. Cell. 2012.
Erratum in
- Cell. 2012 Mar 2;148(5):1066-7
Abstract
Sex hormones such as estrogen and testosterone are essential for sexually dimorphic behaviors in vertebrates. However, the hormone-activated molecular mechanisms that control the development and function of the underlying neural circuits remain poorly defined. We have identified numerous sexually dimorphic gene expression patterns in the adult mouse hypothalamus and amygdala. We find that adult sex hormones regulate these expression patterns in a sex-specific, regionally restricted manner, suggesting that these genes regulate sex typical behaviors. Indeed, we find that mice with targeted disruptions of each of four of these genes (Brs3, Cckar, Irs4, Sytl4) exhibit extremely specific deficits in sex specific behaviors, with single genes controlling the pattern or extent of male sexual behavior, male aggression, maternal behavior, or female sexual behavior. Taken together, our findings demonstrate that various components of sexually dimorphic behaviors are governed by separable genetic programs.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Figure 1. Identification of sexually dimorphic gene expression in the adult mouse brain
(A) Strategy to identify sex differences in gene expression. Boxed areas in Nissl stained coronal sections depict the dissected regions. (B–C“’) Expression of Sytl4 mRNA in serial coronal sections through the forebrain, with more rostral sections on top. Upregulated Sytl4 mRNA in the male BNST (insets). (D) List of genes with sexually dimorphic expression. *, X-linked gene; ‡, imprinted gene. Scale bar = 1 mm. Inset scale bar = 100 µm. See also Figure S1 and Table S1.
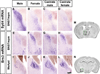
Figure 2. Sexually dimorphic expression of Sytl4 and Brs3
Sytl4 (A–D) and Brs3 (E–L) mRNA expression in coronal sections. Brains from male and female were processed in parallel whereas those from castrate male or female were processed in separate studies and are shown here (and Figure 3) for comparison purposes. (A–D) More Sytl4 mRNA in the male BNSTmpm. (E–L) Less Brs3 mRNA in the male BNSTmpm and MeApd. (M, N) Boxed areas in Nissl stained sections outline the BNST (M) and MeA (N) regions shown in (A–H) and (I–L), respectively. Scale bar (A–L) = 100 µm.
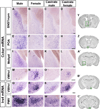
Figure 3. Sexually dimorphic expression of Cckar and Irs4
Cckar (A–P) and Irs4 (Q–X) mRNA expression in coronal sections. (A–L) More Cckar mRNA in the male BNSTmpm, POA, and MeApd. (M–P) More Cckar mRNA in the female VMHvl. (Q–T) More Irs4 mRNA in the VMHvl of the female and castrate female. (U–X) More Irs4 mRNA in the male PMV. (Y, Z, A’–C’) Boxed areas in Nissl stained sections outline the BNST (Y), POA (Z), MeA (A’), VMHvl (B’), and PMV (C’), respectively. Scale bars = 50 µm.
Figure 4. Sexual dimorphism in gene expression and its control by adult sex hormones
Heat map of log10-transformed fold differences in mRNA expression. (A) Individual genes are upregulated in ≥1 brain region in one sex or in distinct regions in both sexes. A brain region can show upregulated expression of distinct genes in both sexes. Red = male-upregulated, green = female-upregulated. (B) Most male-upregulated genes are downregulated after castration. Red = male-upregulated, yellow = no change, green = castrate male-upregulated. (C) Most genes show similar expression in intact and castrate females. Red = female-upregulated, yellow = no change, green = castrate female-upregulated. Heat map scale spans from red to green. Black = not sexually dimorphic or not expressed. p < 0.05 for all changes shown in green or red; p > 0.05 for yellow cells. See also Figures S2, S3.
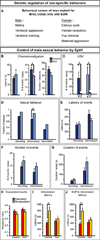
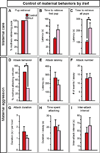
Figure 5. Sytl4 is required for patterning male sexual behavior
(A) Mice mutant for Brs3, Cckar, Irs4, or Sytl4 were tested for deficits in various sex- specific displays. (B) Sytl4−/Y residents (Null) sniff WT female intruders less than Sytl4+/Y residents (Control). (C) All residents vocalize more to WT female than to WT male intruders. (D) Sytl4−/Y residents intromit WT female intruders in more tests. (E–G) The latency, number, and duration of mounts and intromissions are unaffected in Sytl4 mutants. (H) No difference in fraction of mounts that proceed to intromission between males that ejaculate and males that do not. Receptivity index = (# of mounts with intromission)/(# of all mounts). (I, J) Control males that ejaculate show a shorter latency to intromit and proceed faster from the first sniff to the first intromission compared to controls who do not ejaculate. These behavioral parameters are decorrelated with ejaculation in null males. Mean ± SEM; n ≥ 14 animals/genotype; * p < 0.04; ** p < 0.01. See also Figure S4 and Tables S2, S3.
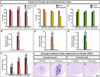
Figure 6. Irs4 is essential for maternal behaviors
(A, B) Irs4−/− (Null) and Irs4−/+ (Control) mothers retrieve pups (A), and the latency to retrieve the first pup is similar between the two groups (B). (C) Irs4 null mothers take longer to retrieve all pups. (D) Fewer Irs4 null mothers attack intruder males. (E–I) When Irs4 null mothers attack intruders they do so similar to control mothers. Mean ± SEM; n ≥11/genotype; * p < 0.04. See also Figure S5, Table S4, and Movies S1, S2.
Figure 7. Cckar is essential for female sexual behavior
(A) WT males mount Cckar null or WT females equivalently, but fewer mutant females allow males to intromit or ejaculate. (B) Lower receptivity index in Cckar−/− females. (C) Lower receptivity index in sexually experienced Cckar−/− females. (D, F) WT males mount, intromit or ejaculate equivalently with females treated with vehicle or Cckar antagonists. (E, G) Both Cckar antagonists reduce sexual receptivity of females. (H–K) Estrogen increases Cckar mRNA in the VMHvl of castrate females. Mean ± SEM; n ≥ 21 (A–C); n ≥ 12/treatment (D–G); n ≥ 3/treatment (H–K); * p < 0.05, ** p < 0.01, *** p ≤ 0.001. Scale bar = 100 µm. See also Figures S5, S6, and Movies S3, S4.
References
- Akesson TR, Mantyh PW, Mantyh CR, Matt DW, Micevych PE. Estrous cyclicity of 125I-cholecystokinin octapeptide binding in the ventromedial hypothalamic nucleus. Evidence for downmodulation by estrogen. Neuroendocrinology. 1987;45:257–262. - PubMed
- Allen E, Doisy EA. An ovarian hormone. Preliminary report on its localization, extraction and partial purification, and action in test animals. Journal of the American Medical Association. 1923;81:819–821. - PubMed
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann. N. Y. Acad. Sci. 2003;1007:176–188. - PubMed
- Babcock AM, Block GJ, Micevych PE. Injections of cholecystokinin into the ventromedial hypothalamic nucleus inhibit lordosis behavior in the rat. Physiol. Behav. 1988;43:195–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases