Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers - PubMed (original) (raw)
doi: 10.1088/1478-3975/9/1/016003. Epub 2012 Feb 3.
Kelly Bethel, Anand Kolatkar, Madelyn S Luttgen, Michael Malchiodi, Franziska Baehring, Katharina Voigt, Daniel Lazar, Jorge Nieva, Lyudmila Bazhenova, Andrew H Ko, W Michael Korn, Ethan Schram, Michael Coward, Xing Yang, Thomas Metzner, Rachelle Lamy, Meghana Honnatti, Craig Yoshioka, Joshua Kunken, Yelena Petrova, Devin Sok, David Nelson, Peter Kuhn
Affiliations
- PMID: 22306768
- PMCID: PMC3387996
- DOI: 10.1088/1478-3975/9/1/016003
Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers
Dena Marrinucci et al. Phys Biol. 2012 Feb.
Abstract
Hematologic spread of carcinoma results in incurable metastasis; yet, the basic characteristics and travel mechanisms of cancer cells in the bloodstream are unknown. We have established a fluid phase biopsy approach that identifies circulating tumor cells (CTCs) without using surface protein-based enrichment and presents them in sufficiently high definition (HD) to satisfy diagnostic pathology image quality requirements. This 'HD-CTC' assay finds >5 HD-CTCs mL(-1) of blood in 80% of patients with metastatic prostate cancer (n = 20), in 70% of patients with metastatic breast cancer (n = 30), in 50% of patients with metastatic pancreatic cancer (n = 18), and in 0% of normal controls (n = 15). Additionally, it finds HD-CTC clusters ranging from 2 HD-CTCs to greater than 30 HD-CTCs in the majority of these cancer patients. This initial validation of an enrichment-free assay demonstrates our ability to identify significant numbers of HD-CTCs in a majority of patients with prostate, breast and pancreatic cancers.
Figures
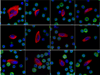
Figure 1
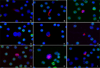
Gallery of representative HD-CTCs found in cancer patients. Each HD-CTC is cytokeratin positive (red), CD45 negative (green), contains a DAPI nucleus (blue), and is morphologically distinct from surrounding white blood cells.
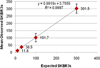
Figure 2
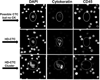
Mean observed SKBR3s plotted against expected SKBR3s. Four aliquots of normal control blood was spiked with varying numbers of SKBR2 cells to produce 4 slides with approximately 10, 30, 100, and 300 cancer cells per slide. The mean of each quadruplicate is displayed as well as error bars noting standard deviation.
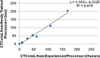
Figure 3
Comparison of CTC counts between two separate processors on 9 different cancer patient samples. CTC/mL counts ranged from 0 to 203.
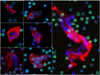
Figure 4
Gallery of representative clusters that are found in most patients with cancer. Clusters range from 2 to over 30 HD-CTCs. Each HD-CTC is cytokeratin positive (red), CD45 negative (green), contains a DAPI nucleus (blue), and is morphologically distinct from surrounding nucleated cells.
Figure 5
Gallery of candidate HD-CTCs that were excluded because they lacked various morphologic or morphometric inclusion criteria. A) Cytokeratin intensity too dim. B) Nuclear size too small. C) Cytokeratin insufficiently circumferential (surrounds less than 2/3 of nucleus) D) Cytokeratin too dim, although appears to be a cluster of two very large cells. E) Nucleus shows apoptotic disintegration changes. F) Nucleus too small and cytoplasm insufficiently circumferential; appears to be a cell in late apoptosis. G) Nucleus too small (same size as surrounding WBC nuclei). H. Cytokeratin present, but not circumferential. I) Cytoplasm insufficiently circumferential, nucleus too small.
Figure 6
Representative images of types of suspected CTCs found in a single prostate cancer patient. Top Row: Suspected CTC that is negative for Cytokeratin and CD45, but has a nucleus that is large and looks like other HD-CTCs found in this patient. Middle Row: Typical HD-CTC that is cytokeratin positive, CD45 negative, with a DAPI nucleus. Bottom Row: HD-CTC cluster of 4 cells.
Similar articles
- Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology.
Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, Marrinucci D, Sandhu A, Perricone A, Thistlethwaite P, Bethel K, Nieva J, Heuvel Mv, Kuhn P. Wendel M, et al. Phys Biol. 2012 Feb;9(1):016005. doi: 10.1088/1478-3967/9/1/016005. Epub 2012 Feb 3. Phys Biol. 2012. PMID: 22307026 Free PMC article. Clinical Trial. - Defining the dimensions of circulating tumor cells in a large series of breast, prostate, colon, and bladder cancer patients.
Mendelaar PAJ, Kraan J, Van M, Zeune LL, Terstappen LWMM, Oomen-de Hoop E, Martens JWM, Sleijfer S. Mendelaar PAJ, et al. Mol Oncol. 2021 Jan;15(1):116-125. doi: 10.1002/1878-0261.12802. Epub 2020 Oct 4. Mol Oncol. 2021. PMID: 32949099 Free PMC article. - Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients.
Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Deng G, et al. Breast Cancer Res. 2008;10(4):R69. doi: 10.1186/bcr2131. Epub 2008 Aug 7. Breast Cancer Res. 2008. PMID: 18687126 Free PMC article. - Circulating tumor cells: clinical validity and utility.
Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Cabel L, et al. Int J Clin Oncol. 2017 Jun;22(3):421-430. doi: 10.1007/s10147-017-1105-2. Epub 2017 Feb 25. Int J Clin Oncol. 2017. PMID: 28238187 Review. - Relevance of CTC Clusters in Breast Cancer Metastasis.
Piñeiro R, Martínez-Pena I, López-López R. Piñeiro R, et al. Adv Exp Med Biol. 2020;1220:93-115. doi: 10.1007/978-3-030-35805-1_7. Adv Exp Med Biol. 2020. PMID: 32304082 Review.
Cited by
- Isolation and retrieval of circulating tumor cells using centrifugal forces.
Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS, Lim WT, Han J, Bhagat AA, Lim CT. Hou HW, et al. Sci Rep. 2013;3:1259. doi: 10.1038/srep01259. Epub 2013 Feb 12. Sci Rep. 2013. PMID: 23405273 Free PMC article. - The prothrombotic activity of cancer cells in the circulation.
Mitrugno A, Tormoen GW, Kuhn P, McCarty OJ. Mitrugno A, et al. Blood Rev. 2016 Jan;30(1):11-9. doi: 10.1016/j.blre.2015.07.001. Epub 2015 Jul 14. Blood Rev. 2016. PMID: 26219246 Free PMC article. Review. - Circulating tumor cells in non-small cell lung carcinoma.
Boshuizen R, Kuhn P, van den Heuvel M. Boshuizen R, et al. J Thorac Dis. 2012 Oct;4(5):456-8. doi: 10.3978/j.issn.2072-1439.2012.08.21. J Thorac Dis. 2012. PMID: 23050106 Free PMC article. - Circulating tumor cells in the early detection of human cancers.
Feng Z, Wu J, Lu Y, Chan YT, Zhang C, Wang D, Luo D, Huang Y, Feng Y, Wang N. Feng Z, et al. Int J Biol Sci. 2022 May 1;18(8):3251-3265. doi: 10.7150/ijbs.71768. eCollection 2022. Int J Biol Sci. 2022. PMID: 35637960 Free PMC article. Review. - Cell State and Cell Type: Deconvoluting Circulating Tumor Cell Populations in Liquid Biopsies by Multi-Omics.
Welter L, Zheng S, Setayesh SM, Morikado M, Agrawal A, Nevarez R, Naghdloo A, Pore M, Higa N, Kolatkar A, Thiele JA, Sharma P, Moore HCF, Richer JK, Elias A, Pienta KJ, Zurita AJ, Gross ME, Shishido SN, Hicks J, Velasco CR, Kuhn P. Welter L, et al. Cancers (Basel). 2023 Aug 3;15(15):3949. doi: 10.3390/cancers15153949. Cancers (Basel). 2023. PMID: 37568766 Free PMC article.
References
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. - PubMed
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical