Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells - PubMed (original) (raw)
Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells
Jordan D T Engbers et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Encoding sensory input requires the expression of postsynaptic ion channels to transform key features of afferent input to an appropriate pattern of spike output. Although Ca(2+)-activated K(+) channels are known to control spike frequency in central neurons, Ca(2+)-activated K(+) channels of intermediate conductance (KCa3.1) are believed to be restricted to peripheral neurons. We now report that cerebellar Purkinje cells express KCa3.1 channels, as evidenced through single-cell RT-PCR, immunocytochemistry, pharmacology, and single-channel recordings. Furthermore, KCa3.1 channels coimmunoprecipitate and interact with low voltage-activated Cav3.2 Ca(2+) channels at the nanodomain level to support a previously undescribed transient voltage- and Ca(2+)-dependent current. As a result, subthreshold parallel fiber excitatory postsynaptic potentials (EPSPs) activate Cav3 Ca(2+) influx to trigger a KCa3.1-mediated regulation of the EPSP and subsequent after-hyperpolarization. The Cav3-KCa3.1 complex provides powerful control over temporal summation of EPSPs, effectively suppressing low frequencies of parallel fiber input. KCa3.1 channels thus contribute to a high-pass filter that allows Purkinje cells to respond preferentially to high-frequency parallel fiber bursts characteristic of sensory input.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
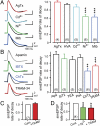
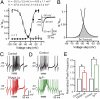
Subthreshold parallel fiber EPSPs generate an AHP consistent with activation of KCa3.1. All records were evoked using simEPSCs to test postsynaptic channel contributions, with drug effects on the rate of EPSP decay normalized to the control 5 mV simEPSP. (A and B) Representative recordings (Left) and bar plots (Right) showing the effects of Ca2+ and K+ channel blockers. (A) The simEPSP rate of decay is not significantly affected by AgTx (200 nM) or other HVA Ca2+ channel blockers (ω-conotoxin GVIA, 1 μM; nifedipine, 1 μM; SNX-482, 200 nM) or Cd2+ (30 μM), but is reduced by putative T-type Ca2+ channel blockers Ni2+ (100 μM) and mibefradil (Mib,1 μM). (B) The simEPSP rate of decay is unaffected by the KCa2.x blocker apamin (100 nM), or KCa1.1 blockers IbTx (200 nM), TEA (5 mM), or paxilline (100 nM), but is significantly reduced by ChTx (100 nM) and TRAM-34 (100 nM). (C) Internal dialysis of Camstatin (5 μM) occludes the effect of TRAM-34 on the simEPSP rate of decay. (D) Pretreatment with Ni2+ occludes the action of TRAM-34 on the simEPSP rate of decay. Sample numbers are shown in brackets at the base of bar graphs. Average values are mean ± SEM; **P < 0.01, ***P < 0.001.
Fig. 2.
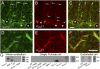
Purkinje cells express KCa3.1 channels that colocalize with Cav3.2 protein. (A_–_C) Dual-label immunocytochemistry for Cav3.2 (A) and KCa3.1 (B) in a coronal section reveals protein colocalized (arrows) at the soma (asterisks) and restricted segments of dendritic branches (C). (D_–_F) High-power view of Purkinje cell dendrites in a sagittal section dual-labeled for Cav3.2 (D) and KCa3.1 protein (E), with overlay (F) illustrating colocalization over specific segments of dendritic branches. (G) RT-PCR reveals KCa3.1 and MRF-1 mRNA in whole cerebellum (Left), and KCa1.1, KCa2.2, and KCa3.1 but not MRF-1 in single Purkinje cell cytoplasmic extracts (Center). The KCa3.1 product in Purkinje cells matches that found in endothelial cells (Right). (Scale bars, 20 μm.)
Fig. 3.
Purkinje cells express Ca2+-activated intermediate conductance K+ channels. (A) Spontaneous on-cell single-channel recordings from three different cells during a steady-state (5 min) pipette holding potential of 30 mV. Open (o) and closed (c) states are indicated. (B) Plot of mean single-channel amplitudes in on-cell recordings at steady-state potentials up to +30 mV reveals a mean conductance of 36.3 pS (n = 5). (C) (Left) Long-duration on-cell channel recordings (+30 mV pipette potential) before and after perfusing the Ca2+ ionophore A23187 (2 μM) and block by TRAM-34 (100 nM) (n = 6). (Right) Bar plots show channel open probability under the conditions shown in the channel recordings (n = 6). Average values are mean ± SEM; *P < 0.05; **P < 0.01.
Fig. 4.
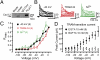
KCa3.1 channel activation is coupled to Cav3-mediated Ca2+ influx. (A) Western blot showing coimmunoprecipitation of Cav3.2 and KCa3.1 channels from cerebellar lysate. (B) Outside-out recordings from separate Purkinje cell somata in response to steps from −110 mV to 0 mV. Shown are currents calculated as the difference from those evoked at a −40 mV holding potential, or blocked by TRAM-34 (100 nM) or Ni2+ (100 μM). (C) Mean I-V plots for currents isolated as in B indicate a common activation in the low voltage range. (D) Mean I-V plots of TRAM-34-sensitive currents in outside-out recordings with either high EGTA or BAPTA in the electrode. All recordings were obtained from the somata of P 18–25 Purkinje cells in 1.5 mM external Ca2+. Average values are mean ± SEM.
Fig. 5.
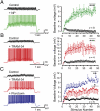
The Cav3–KCa3.1 complex regulates temporal summation of parallel fiber EPSPs. (A and B) Representative recordings and plots of the baseline membrane voltage during 25-Hz trains of parallel fiber-evoked EPSPs before and after applying Ni2+ (A, 100 μM, green) or TRAM-34 (B, 100 nM, red). Stimulus intensity was adjusted to evoke an initial EPSP of 2 mV. (C) Recordings and plots of baseline voltage during 25 Hz parallel fiber stimulus trains in a coronal slice in the absence of picrotoxin to preserve feed-forward inhibition. TRAM-34 (red) substantially increases temporal summation, with an additional increase upon addition of picrotoxin (50 μM, blue) to block GABAergic inhibition. Statistical significance tested for last 10 pulses of stimulus trains in A_–_C (indicated by bars). Spikes were truncated in A_–_C and average values are mean ± SEM; *P < 0.05; **P < 0.01, ***P < 0.001.
Fig. 6.
Cav3 window current straddles spike threshold in Purkinje cells and the Cav3–KCa3.1 complex affects EPSP summation under physiological conditions. (A) Mean conductance and inactivation plots calculated for whole-cell Cav3 current recorded from P10–12 Purkinje cells (Inset). (B) Expanded view of the fits for activation and inactivation curves shown in A reveal that Cav3 window current (gray) in relation to spike threshold (dashed line). (C_–_E) The effects of blocking the Cav3–KCa3.1 complex during a five-pulse train of parallel fiber stimulation (100 Hz, arrows). Resting potential was depolarized to a level sufficient to evoke ∼50-Hz tonic firing, with spike threshold indicated at Left. Blocking the Cav3–KCa3.1 complex with either TRAM-34 (C, 100 nM, red) or Ni2+ (D, 100 μM, green) reveals substantial control over EPSP summation and spike frequency (E). Spikes were truncated for display in C and D, and picrotoxin (50 μM) was present for all tests in C_–_E. All average values are mean ± SEM; *P < 0.05.
Similar articles
- Two Distinct Sets of Ca2+ and K+ Channels Are Activated at Different Membrane Potentials by the Climbing Fiber Synaptic Potential in Purkinje Neuron Dendrites.
Ait Ouares K, Filipis L, Tzilivaki A, Poirazi P, Canepari M. Ait Ouares K, et al. J Neurosci. 2019 Mar 13;39(11):1969-1981. doi: 10.1523/JNEUROSCI.2155-18.2018. Epub 2019 Jan 10. J Neurosci. 2019. PMID: 30630881 Free PMC article. - Signal processing by T-type calcium channel interactions in the cerebellum.
Engbers JD, Anderson D, Zamponi GW, Turner RW. Engbers JD, et al. Front Cell Neurosci. 2013 Nov 27;7:230. doi: 10.3389/fncel.2013.00230. Front Cell Neurosci. 2013. PMID: 24348329 Free PMC article. Review. - The Origin of Physiological Local mGluR1 Supralinear Ca2+ Signals in Cerebellar Purkinje Neurons.
Ait Ouares K, Canepari M. Ait Ouares K, et al. J Neurosci. 2020 Feb 26;40(9):1795-1809. doi: 10.1523/JNEUROSCI.2406-19.2020. Epub 2020 Jan 22. J Neurosci. 2020. PMID: 31969470 Free PMC article. - An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses.
De Schutter E, Bower JM. De Schutter E, et al. J Neurophysiol. 1994 Jan;71(1):401-19. doi: 10.1152/jn.1994.71.1.401. J Neurophysiol. 1994. PMID: 8158238 - SK2 channel expression and function in cerebellar Purkinje cells.
Hosy E, Piochon C, Teuling E, Rinaldo L, Hansel C. Hosy E, et al. J Physiol. 2011 Jul 15;589(Pt 14):3433-40. doi: 10.1113/jphysiol.2011.205823. Epub 2011 Apr 26. J Physiol. 2011. PMID: 21521760 Free PMC article. Review.
Cited by
- Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes.
Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW. Rehak R, et al. PLoS One. 2013 Apr 23;8(4):e61844. doi: 10.1371/journal.pone.0061844. Print 2013. PLoS One. 2013. PMID: 23626738 Free PMC article. - Cav1.3 Channels as Key Regulators of Neuron-Like Firings and Catecholamine Release in Chromaffin Cells.
Vandael DH, Marcantoni A, Carbone E. Vandael DH, et al. Curr Mol Pharmacol. 2015;8(2):149-61. doi: 10.2174/1874467208666150507105443. Curr Mol Pharmacol. 2015. PMID: 25966692 Free PMC article. Review. - Long-Term Potentiation at the Mossy Fiber-Granule Cell Relay Invokes Postsynaptic Second-Messenger Regulation of Kv4 Channels.
Rizwan AP, Zhan X, Zamponi GW, Turner RW. Rizwan AP, et al. J Neurosci. 2016 Nov 2;36(44):11196-11207. doi: 10.1523/JNEUROSCI.2051-16.2016. J Neurosci. 2016. PMID: 27807163 Free PMC article. - Genetic KCa3.1-deficiency produces locomotor hyperactivity and alterations in cerebral monoamine levels.
Lambertsen KL, Gramsbergen JB, Sivasaravanaparan M, Ditzel N, Sevelsted-Møller LM, Oliván-Viguera A, Rabjerg M, Wulff H, Köhler R. Lambertsen KL, et al. PLoS One. 2012;7(10):e47744. doi: 10.1371/journal.pone.0047744. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077667 Free PMC article. - Inhibition promotes long-term potentiation at cerebellar excitatory synapses.
Binda F, Dorgans K, Reibel S, Sakimura K, Kano M, Poulain B, Isope P. Binda F, et al. Sci Rep. 2016 Sep 19;6:33561. doi: 10.1038/srep33561. Sci Rep. 2016. PMID: 27641070 Free PMC article.
References
- D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: Focus on the granular layer. Trends Neurosci. 2009;32:30–40. - PubMed
- Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: From protein complexes to function. Physiol Rev. 2010;90:1437–1459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous