YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy - PubMed (original) (raw)
YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy
Alexander von Gise et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Heart growth is tightly controlled so that the heart reaches a predetermined size. Fetal heart growth occurs through cardiomyocyte proliferation, whereas postnatal heart growth involves primarily physiological cardiomyocyte hypertrophy. The Hippo kinase cascade is an important regulator of organ growth. A major target of this kinase cascade is YAP1, a transcriptional coactivator that is inactivated by Hippo kinase activity. Here, we used both genetic gain and loss of Yap1 function to investigate its role in regulating proliferative and physiologic hypertrophic heart growth. Fetal Yap1 inactivation caused marked, lethal myocardial hypoplasia and decreased cardiomyocyte proliferation, whereas fetal activation of YAP1 stimulated cardiomyocyte proliferation. Enhanced proliferation was particularly dramatic in trabecular cardiomyocytes that normally exit from the cell cycle. Remarkably, YAP1 activation was sufficient to stimulate proliferation of postnatal cardiomyocytes, both in culture and in the intact heart. A dominant negative peptide that blocked YAP1 binding to TEAD transcription factors inhibited YAP1 proliferative activity, indicating that this activity requires YAP1-TEAD interaction. Although Yap1 was a critical regulator of cardiomyocyte proliferation, it did not influence physiological hypertrophic growth of cardiomyocytes, because postnatal Yap1 gain or loss of function did not significantly alter cardiomyocyte size. These studies demonstrate that Yap1 is a crucial regulator of cardiomyocyte proliferation, cardiac morphogenesis, and myocardial trabeculation. Activation of Yap1 in postnatal cardiomyocytes may be a useful strategy to stimulate cardiomyocyte expansion in therapeutic myocardial regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
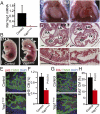
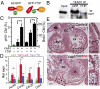
Fetal cardiomyocyte-restricted inactivation of YAP1. (A) YAP1 mRNA levels in control (Yap1fl/+::Tnnt2–Cre::Rosa26mTmG/+) and Yap1Tnnt2 (Yap1fl/fl::Tnnt2–Cre::Rosa26mTmG/+) mutant cardiomyocytes, isolated at E12.5 by FACS for GFP. (B) Hydrops fetalis and peripheral hemorrhage in mutant embryo at E13.5. (Scale bar: 500 μm.) (C) Whole-mount image of E16.5 embryos after removal of the ventral chest wall, showing severe hypoplasia of mutant hearts. Hypoplasia was more severe in the left ventricle. For clarity, the white line indicates the edge of the heart. (Scale bar: 500 μm.) (D) Histological sections of E11.5 hearts. Magnifications show dramatic hypoplasia of compact myocardium in mutants (black brackets). (Scale bar: 100 μm.) (E and F) Reduced pH3 immunoreactivity in Yap1Tnnt2 mutant hearts. n = 3. (Scale bar in E: 10 μm.) (G and H) Reduced EdU staining in Yap1Tnnt2 mutant hearts. n = 3. (Scale bar: 10 μm.)
Fig. 2.
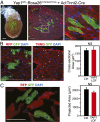
YAP1 is not cell autonomously required for postnatal hypertrophic cardiomyocyte growth. (A) Mosaic Cre-mediated recombination after retro-orbital delivery of Ad:Tnnt2–Cre to P3 neonatal Yap1fl/fl::Rosa26mTmG/mTmG mice. Boxed area is magnified in the Right panel. [Scale bars (from left): 500, 100, 10 μm.] (B and C) Hearts were examined at 4 wk (B) or 6 wk (C). Sizes of YAP1-deficient (mGFP+ and mRFP−) and control (mGFP− and mRFP+) cardiomyocytes were measured in histological sections (cross-sectional area, B) or in dissociated preparations (projected area, C). No significant difference was detected between groups (NS; n = 4). (Scale bars: 100 μm.)
Fig. 3.
Activated YAP1 stimulated proliferation of cultured cardiomyocytes. (A) aYAP1 stimulated cell-cycle activity of P4 neonatal rat cardiomyocytes, as measured by immunostaining for BrdU, pH3, and Aurora B (AurB). (Scale bars: 50 μm.) (B) Sequential imaging of cardiomyocytes at the indicated times after aYAP1 transduction. Two cardiomyocytes underwent cytokinesis in this field (arrowheads). At 60 h, cultures were immunostained for the cardiomyocyte marker TNNI3. Full imaging series is in
Fig. S5_D_
. *P = 0.015.
Fig. 4.
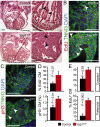
Fetal YAP1 gain of function stimulated cardiomyocyte proliferation in vivo. (A) Histological sections of Yap1GOF heart at E12.5 revealed marked hypertrabeculation that nearly obliterated the cardiac chambers. Black arrowheads indicate trabecular myocardium. (B and C) pH3 and EdU staining showed elevated cardiomyocyte proliferation, particularly in trabecular myocardium (white arrowheads). (D) Quantitation of B and C for ventricular myocardium (compact and trabecular pooled). (E) Quantitation of cardiomyocyte EdU uptake in compact vs. trabecular myocardium. (Scale bars: 100 μm.)
Fig. 5.
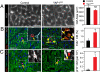
Postnatal YAP1 gain of function stimulated cardiomyocyte proliferation in vivo. YAP1GOF or control mice were treated with Dox from P5 to P15, when they were analyzed for cardiomyocyte proliferation. (A) The size of YAP1 gain-of-function cardiomyocytes was indistinguishable from control. Wheat germ agglutinin (WGA) staining outlined the edges of cardiomyocytes. (B and C) Cardiomyocyte proliferation was increased based on EdU labeling index (B, deconvolution) and pH3 staining (C, confocal). Arrowheads indicate cardiomyocytes and arrows nonmyocytes. Insets show magnifications of boxed regions, with cardiomyocyte borders highlighted by WGA staining. n = 3. *P < 0.05. (Scale bars: 20 μm.)
Fig. 6.
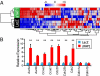
YAP1 promotes expression of cell-cycle genes through interaction with TEAD1. (A) Heat map displaying two-way hierarchical clustering of cell-cycle genes in P4 neonatal cardiomyocytes expressing either aYAP1 or LacZ (control). Expression of cell-cycle genes segregated samples into treatment groups. (B) Validation of differential expression of cell-cycle genes by qRT-PCR. **P < 0.001. n = 4.
Fig. 7.
YAP1 stimulation of cardiomyocyte proliferation requires TEAD interaction. (A) YAP1-TEAD inhibitory peptide (YTIP) strategy. (B) Endogenous TEAD1 and YAP1 interaction was blocked by Ad:GFP–YTIP in MES13 cells. (C) aYAP1 stimulation of P4 neonatal cardiomyocyte proliferation was attenuated by GFP–YTIP, as measured by BrdU and pH3 immunostaining. *P < 0.01. n = 3. (D) Inhibition of TEAD interaction reduced expression of YAP1-activated cell-cycle genes, as measured by qRT-PCR. *P < 0.05. n = 3. (E) Cardiomyocyte YAP1S79A, deficient in TEAD interaction, did not support normal fetal myocardial growth. Yap1Tnnt2/S79A indicates the genotype Yap1fl/S79A::Tnnt2–Cre. H&E-stained sections of E12.5 heart are shown. (Right) Boxed areas are enlarged. Brackets indicate compact myocardial thickness. (Scale bars: 100 μm.)
Similar articles
- Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury.
Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Del Re DP, et al. J Biol Chem. 2013 Feb 8;288(6):3977-88. doi: 10.1074/jbc.M112.436311. Epub 2012 Dec 30. J Biol Chem. 2013. PMID: 23275380 Free PMC article. - Hippo Deficiency Leads to Cardiac Dysfunction Accompanied by Cardiomyocyte Dedifferentiation During Pressure Overload.
Ikeda S, Mizushima W, Sciarretta S, Abdellatif M, Zhai P, Mukai R, Fefelova N, Oka SI, Nakamura M, Del Re DP, Farrance I, Park JY, Tian B, Xie LH, Kumar M, Hsu CP, Sadayappan S, Shimokawa H, Lim DS, Sadoshima J. Ikeda S, et al. Circ Res. 2019 Jan 18;124(2):292-305. doi: 10.1161/CIRCRESAHA.118.314048. Circ Res. 2019. PMID: 30582455 Free PMC article. - Hippo signaling impedes adult heart regeneration.
Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Heallen T, et al. Development. 2013 Dec;140(23):4683-90. doi: 10.1242/dev.102798. Development. 2013. PMID: 24255096 Free PMC article. - Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration.
Flinn MA, Link BA, O'Meara CC. Flinn MA, et al. Semin Cell Dev Biol. 2020 Apr;100:11-19. doi: 10.1016/j.semcdb.2019.09.004. Epub 2019 Oct 9. Semin Cell Dev Biol. 2020. PMID: 31606277 Free PMC article. Review. - The hippo pathway in heart development, regeneration, and diseases.
Zhou Q, Li L, Zhao B, Guan KL. Zhou Q, et al. Circ Res. 2015 Apr 10;116(8):1431-47. doi: 10.1161/CIRCRESAHA.116.303311. Circ Res. 2015. PMID: 25858067 Free PMC article. Review.
Cited by
- Increased Reactive Oxygen Species-Mediated Ca2+/Calmodulin-Dependent Protein Kinase II Activation Contributes to Calcium Handling Abnormalities and Impaired Contraction in Barth Syndrome.
Liu X, Wang S, Guo X, Li Y, Ogurlu R, Lu F, Prondzynski M, de la Serna Buzon S, Ma Q, Zhang D, Wang G, Cotton J, Guo Y, Xiao L, Milan DJ, Xu Y, Schlame M, Bezzerides VJ, Pu WT. Liu X, et al. Circulation. 2021 May 11;143(19):1894-1911. doi: 10.1161/CIRCULATIONAHA.120.048698. Epub 2021 Apr 1. Circulation. 2021. PMID: 33793303 Free PMC article. - Neuregulin-1, a potential therapeutic target for cardiac repair.
Wang Y, Wei J, Zhang P, Zhang X, Wang Y, Chen W, Zhao Y, Cui X. Wang Y, et al. Front Pharmacol. 2022 Aug 31;13:945206. doi: 10.3389/fphar.2022.945206. eCollection 2022. Front Pharmacol. 2022. PMID: 36120374 Free PMC article. Review. - Yes-Associated Protein and Transcriptional Coactivator with PDZ-Binding Motif in Cardiovascular Diseases.
Li R, Huang W. Li R, et al. Int J Mol Sci. 2023 Jan 14;24(2):1666. doi: 10.3390/ijms24021666. Int J Mol Sci. 2023. PMID: 36675179 Free PMC article. Review. - Hippo-Yap signaling in cardiac and fibrotic remodeling.
Del Re DP. Del Re DP. Curr Opin Physiol. 2022 Apr;26:100492. doi: 10.1016/j.cophys.2022.100492. Epub 2022 Apr 29. Curr Opin Physiol. 2022. PMID: 36644337 Free PMC article. - The Hippo effector YAP1/TEAD1 regulates EPHA3 expression to control cell contact and motility.
Al-Mathkour MM, Dwead AM, Alp E, Boston AM, Cinar B. Al-Mathkour MM, et al. Sci Rep. 2022 Mar 9;12(1):3840. doi: 10.1038/s41598-022-07790-4. Sci Rep. 2022. PMID: 35264657 Free PMC article.
References
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res. 2003;93:857–865. - PubMed
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. - PubMed
- Lai ZC, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials