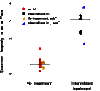Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis - PubMed (original) (raw)
Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis
Heidi H Kong et al. Genome Res. 2012 May.
Abstract
Atopic dermatitis (AD) has long been associated with Staphylococcus aureus skin colonization or infection and is typically managed with regimens that include antimicrobial therapies. However, the role of microbial communities in the pathogenesis of AD is incompletely characterized. To assess the relationship between skin microbiota and disease progression, 16S ribosomal RNA bacterial gene sequencing was performed on DNA obtained directly from serial skin sampling of children with AD. The composition of bacterial communities was analyzed during AD disease states to identify characteristics associated with AD flares and improvement post-treatment. We found that microbial community structures at sites of disease predilection were dramatically different in AD patients compared with controls. Microbial diversity during AD flares was dependent on the presence or absence of recent AD treatments, with even intermittent treatment linked to greater bacterial diversity than no recent treatment. Treatment-associated changes in skin bacterial diversity suggest that AD treatments diversify skin bacteria preceding improvements in disease activity. In AD, the proportion of Staphylococcus sequences, particularly S. aureus, was greater during disease flares than at baseline or post-treatment, and correlated with worsened disease severity. Representation of the skin commensal S. epidermidis also significantly increased during flares. Increases in Streptococcus, Propionibacterium, and Corynebacterium species were observed following therapy. These findings reveal linkages between microbial communities and inflammatory diseases such as AD, and demonstrate that as compared with culture-based studies, higher resolution examination of microbiota associated with human disease provides novel insights into global shifts of bacteria relevant to disease progression and treatment.
Figures
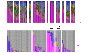
Figure 1.
AD disease severity. (A) Representative clinical images of the antecubital (Ac, left) and popliteal creases (Pc, right) in two patients with overall disease severity scores (objective SCORAD). (B) Longitudinal objective SCORAD trend for each patient (n = 12) at baseline, flare, and postflare.
Figure 2.
Microbial community-level statistics in the AD microbiome. (A) Relationship between objective SCORAD and Shannon diversity in the Ac of AD patients. Partial correlation (adjusting for disease state). (B) Longitudinal Shannon diversity trend in AD grouped by no-treatment (trt) and intermittent-trt flares (n = 12, Ac). (C) Mean Shannon diversity ± SEM in controls and all AD disease states (Ac, Pc, volar forearm [Vf], nares [_N_]). (D) Mean theta (θ) similarity coefficients ± SEM. Pairwise comparisons of community structure between individuals within a control or AD disease group. Bars represent average of all pairwise comparisons of community structure for control individuals to other controls, baselines to baselines, flares(no-trt)-flares(no-trt), flare(intermittent-trt)-flare(intermittent-trt), and postflares-postflares.
Figure 3.
Bacterial taxonomic classifications in the AD skin microbiome. (A) Mean relative abundance of the 14 major phyla-order in the antecubital (Ac) and popliteal creases (Pc) for controls and AD disease states: baseline, flare (no-treatment [trt] and intermittent-trt), and postflare (Supplemental Table S13 for order of subjects). (B) Mean relative abundances for Ac and Pc of species-level classifications of staphylococcal species. Order of subjects follows A.
Figure 4.
Relationship between staphylococcal species and AD. (A) Longitudinal trend of mean proportion of S. aureus in AD in antecubital and popliteal creases (AcPc, n = 12) grouped by no-treatment (trt) and intermittent-trt flares. (B) Proportion of S. aureus and Shannon diversity index in AcPc. Partial correlation (adjusting for disease state, AcPc). (C) Longitudinal trend of mean proportion of S. epidermidis in AcPc. (D) Correlation of proportion of S. aureus versus objective SCORAD for each site (Ac, Pc, Volar forearm/Vf, Nares/N). Partial correlation (adjusting for disease state and site).
Figure 5.
Shannon diversity index during flares. Circles indicate AD patients with a full longitudinal cycle (baseline, flare, and postflare). Triangles indicate flares from four additional (add'l) patients. Means between no-treatment (trt) and intermittent-trt tested with Wilcoxon rank-sum test (P < 0.0025, n = 16).
Figure 6.
AD microbiome progression hypothesis. (*) Proposed relationship among shifts in skin microbial diversity, the proportion of Staphylococcus, and disease severity.
Similar articles
- Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year.
Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, Jo JH, Segre JA, Kong HH, Irvine AD. Kennedy EA, et al. J Allergy Clin Immunol. 2017 Jan;139(1):166-172. doi: 10.1016/j.jaci.2016.07.029. Epub 2016 Sep 5. J Allergy Clin Immunol. 2017. PMID: 27609659 Free PMC article. - Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus.
Bjerre RD, Holm JB, Palleja A, Sølberg J, Skov L, Johansen JD. Bjerre RD, et al. BMC Microbiol. 2021 Sep 23;21(1):256. doi: 10.1186/s12866-021-02302-2. BMC Microbiol. 2021. PMID: 34551705 Free PMC article. - Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration.
Baurecht H, Rühlemann MC, Rodríguez E, Thielking F, Harder I, Erkens AS, Stölzl D, Ellinghaus E, Hotze M, Lieb W, Wang S, Heinsen-Groth FA, Franke A, Weidinger S. Baurecht H, et al. J Allergy Clin Immunol. 2018 May;141(5):1668-1676.e16. doi: 10.1016/j.jaci.2018.01.019. Epub 2018 Feb 5. J Allergy Clin Immunol. 2018. PMID: 29421277 - Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review.
Demessant-Flavigny AL, Connétable S, Kerob D, Moreau M, Aguilar L, Wollenberg A. Demessant-Flavigny AL, et al. J Eur Acad Dermatol Venereol. 2023 Jun;37 Suppl 5:3-17. doi: 10.1111/jdv.19125. J Eur Acad Dermatol Venereol. 2023. PMID: 37232427 Review. - Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis.
Özdemіr E, Öksüz L. Özdemіr E, et al. Arch Microbiol. 2024 Sep 20;206(10):410. doi: 10.1007/s00203-024-04134-w. Arch Microbiol. 2024. PMID: 39302484 Review.
Cited by
- Identification of cutaneous fungi and mites in adult atopic dermatitis: analysis by targeted 18S rRNA amplicon sequencing.
Edslev SM, Andersen PS, Agner T, Saunte DML, Ingham AC, Johannesen TB, Clausen ML. Edslev SM, et al. BMC Microbiol. 2021 Mar 4;21(1):72. doi: 10.1186/s12866-021-02139-9. BMC Microbiol. 2021. PMID: 33663381 Free PMC article. - Targeting IL-4 for the Treatment of Atopic Dermatitis.
Chiricozzi A, Maurelli M, Peris K, Girolomoni G. Chiricozzi A, et al. Immunotargets Ther. 2020 Sep 29;9:151-156. doi: 10.2147/ITT.S260370. eCollection 2020. Immunotargets Ther. 2020. PMID: 33062619 Free PMC article. Review. - What's New in Topicals for Atopic Dermatitis?
Kleinman E, Laborada J, Metterle L, Eichenfield LF. Kleinman E, et al. Am J Clin Dermatol. 2022 Sep;23(5):595-603. doi: 10.1007/s40257-022-00712-0. Epub 2022 Sep 1. Am J Clin Dermatol. 2022. PMID: 36048410 Free PMC article. Review. - Managing the Skin Microbiome as a New Bacteriotherapy for Inflammatory Atopic Dermatitis.
Dewi DAR, Perdiyana A, Wiliantari NM, Nadhira F, Arkania N, Salsabila CA, Allun CV, Allatib A, Dewantara K. Dewi DAR, et al. Cureus. 2023 Nov 14;15(11):e48803. doi: 10.7759/cureus.48803. eCollection 2023 Nov. Cureus. 2023. PMID: 38024036 Free PMC article. Review. - The absence of a microbiota enhances TSLP expression in mice with defective skin barrier but does not affect the severity of their allergic inflammation.
Yockey LJ, Demehri S, Turkoz M, Turkoz A, Ahern PP, Jassim O, Manivasagam S, Kearney JF, Gordon JI, Kopan R. Yockey LJ, et al. J Invest Dermatol. 2013 Dec;133(12):2714-2721. doi: 10.1038/jid.2013.228. Epub 2013 May 22. J Invest Dermatol. 2013. PMID: 23698100 Free PMC article.
References
- Archer GL, Climo MW 2001. Staphylococcus aureus bacteremia–consider the source. N Engl J Med 344: 55–56 - PubMed
- Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. 1995. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 8: 483–491 - PubMed
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368: 733–743 - PubMed
- Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC 2010. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 163: 12–26 - PubMed
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T 2006. The burden of skin diseases: 2004: A joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 55: 490–500 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases