TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages - PubMed (original) (raw)
TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages
Christian P Bauerfeld et al. J Immunol. 2012.
Abstract
Mitochondria play a critical role in cell survival and death. Mitochondrial recovery during inflammatory processes such as sepsis is associated with cell survival. Recovery of cellular respiration, mitochondrial biogenesis, and function requires coordinated expression of transcription factors encoded by nuclear and mitochondrial genes, including mitochondrial transcription factor A (T-fam) and cytochrome c oxidase (COX, complex IV). LPS elicits strong host defenses in mammals with pronounced inflammatory responses, but also triggers activation of survival pathways such as AKT pathway. AKT/PKB is a serine/threonine protein kinase that plays an important role in cell survival, protein synthesis, and controlled inflammation in response to TLRs. Hence we investigated the role of LPS-mediated AKT activation in mitochondrial bioenergetics and function in cultured murine macrophages (B6-MCL) and bone marrow-derived macrophages. We show that LPS challenge led to increased expression of T-fam and COX subunits I and IV in a time-dependent manner through early phosphorylation of the PI3K/AKT pathway. PI3K/AKT pathway inhibitors abrogated LPS-mediated T-fam and COX induction. Lack of induction was associated with decreased ATP production, increased proinflammatory cytokines (TNF-α), NO production, and cell death. The TLR4-mediated AKT activation and mitochondrial biogenesis required activation of adaptor protein MyD88 and Toll/IL-1R domain-containing adaptor-inducing IFN-β. Importantly, using a genetic approach, we show that the AKT1 isoform is pivotal in regulating mitochondrial biogenesis in response to TLR4 agonist.
Figures
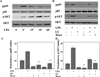
Figure 1. Time dependent effect of LPS on mitochondrial transcription factors (NRF-1, T-fam) and cytochrome c oxidase subunit I and IV
Murine macrophages (B6-MCL) were cultured in a density of 2×106 per well 24 h prior to LPS treatment. Cells were treated with LPS (100 ng/mL) for different time periods. A. Whole cell extracts were prepared and 30 µg of total protein were subjected to SDS-PAGE and Western blot analysis using antibodies against COX subunit I, IV and NRF-1 and T-fam. Equal loading was determined using β actin. Cells responded to LPS stimulation with an early increase in expression of T-fam followed by increased expression of both COX subunits (I and IV). NRF-1 increased at a later time point (24h). B. Densitometric analysis (mean) of NRF-1, COX I and IV, and T-fam of 4 independent experiments. C. Relative gene expression for T-fam, COX IV and NRF-1 in response to LPS. Cells were treated with LPS (100ng/mL) for 2h, RNA was isolated and qRT-PCR was carried out for T-fam, NRF-1 and COX IV. Values were normalized to β actin. Results represent mean values of 4 independent experiments each performed in triplicates. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001.
Figure 2. LPS effect on mitochondrial membrane potential, COX activity and ATP production
A. As control cells were treated either with FCCP (1µM) to dissipate ΔΨm resulting in a left shift of TMRM fluorescence or treated with 0.5 µM oligomycin to increase ΔΨm showing a right shift of fluorescence probe. All measurements were performed with a FACScan (Becton-Dickinson) flow cytometer equipped with a yellow fluorescence (excitation, 532 nm laser; emission, 585 nm; band pass, 42 nm) analyzing 10,000 cells in each run. Data obtained were analyzed with the Cell Quest software (n = 4). TMRM fluorescence was normalized to MitoTracker red fluorescence, a membrane potential-independent mitochondrial marker in these cells. B, C and D. Kinetic of ΔΨm in response to LPS treatment. Cells were incubated with LPS (100 ng/mL), and relative membrane potentials were determined using the fluorescent probe TMRM. 30 min LPS treatment led to an early decrease of ΔΨm (B) and recovery starting after 3h treatment (C). After 6h of LPS treatment cells showed an increase in fluorescent signal suggesting recovery of mitochondrial mass and function (D). E. LPS increased COX activity. B6-MCL cells were cultured with or without LPS (100ng/mL). COX activity was measured 6h after LPS treatment in solubilized cells by the addition of increasing amounts of cytochrome c. Specific activity (TN, turnover number) is defined as consumed O2 ((µmol)/(min⋅total protein (mg)). Shown are representative results of three independent experiments. F. Time dependent changes of ATP levels in response to LPS. B6-MCL cells were incubated with LPS (100 ng/mL) for different time points as indicated. ATP concentrations were measured using the bioluminescence method. As indicated LPS treatment led to a time-dependent increase of ATP concentration. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. ** expresses a p-value of <0.001.
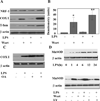
Figure 3. LPS treatment activates the PI3 kinase/AKT pathway in murine macrophages
A. Murine macrophages (B6-MCL) were cultured and treated with LPS (100 ng/mL) for different time points. Total cell lysates were prepared and 20 µg of total cellular proteins were subjected to SDS-PAGE and Western blot analysis using phosphoepitope-specific PI3K p85 (Tyr 458/p55 Tyr199) and AKT (Ser 473) antibodies. Equal loading of protein was confirmed using total p85 and AKT antibodies. The results shown are representative of three independent experiments. LPS treatment led to an early phosphorylation of the PI3K p85/AKT pathway. B. Murine macrophages (B6-MCL) were treated with LY294002 (50 ng/mL) or wortmannin (100 ng/mL) 30 min prior to LPS (100 ng/mL) stimulation. Thirty min after LPS stimulation, total cell lysates were prepared and 20 µg of total cellular protein were subjected to Western blot analysis using phosphoepitope-specific PI3K detecting dual phosphorylation sites on p85 (Tyr 458/ p55 Tyr199) and AKT (Ser 473). Equal loading of protein was confirmed using total AKT and p85 antibodies. Both inhibitors blocked the effect of LPS on phosphorylation of p85 and AKT. Panel C and D are the densitometric analyses (mean ± SEM) of phosphorylated form of p85 and AKT in response to LPS treatment in presence and absence of wortmannin and LY294002. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001.
Figure 4. Inhibition of the PI3 kinase/AKT pathway abrogates LPS-mediated upregulation of COX I and T-fam but not manganese superoxide dismutase
A. Murine macrophages (B6-MCL) were pretreated with wortmannin (100 ng/mL) for 30 min prior to adding LPS (100 ng/mL) to the media for 6 h. After incubation, whole cell extracts were prepared and 20 µg of total protein was subjected to SDS-PAGE and Western blot analysis for NRF-1, COX I, T-fam, and β actin as loading control. B. Densitometric analysis (mean ±SEM) of COX I in 4 independent experiments in response to LPS stimulation in the presence of wortmannin. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001. C. Murine macrophages (B6-MCL) were pretreated with okadaic acid (10 nM) 30 min prior to adding LPS (100 ng/mL) to the media for 6 h. After incubation, whole cell extracts were prepared and 20 µg of total protein were subjected to SDS-PAGE and Western blot analysis using an antibody against COX I, β actin was used to confirm equal loading. The figure is a representative of four independent experiments. D. Upper panel. Time dependent expression of MnSOD in B6-MCL cells. Cells were treated with LPS (100 ng/mL) for different time periods as indicated. Total cell lysates were prepared and 20 µg of protein was subjected to SDS-PAGE and Western blot analysis using an antibody against MnSOD. D. Lower panel. Murine macrophages (B6-MCL) were pretreated with wortmannin (100 ng/mL) or LY294002 (50 ng/mL) for 30 min prior to adding LPS (100 ng/mL) to the media for 6 h. After incubation, whole cell extracts were prepared and 20 µg of total protein was subjected to SDS-PAGE and Western blot analysis using an antibody against MnSOD. Equal loading was assessed using a β actin antibody. The presence of wortmannin or LY294002 did not abrogate LPS-mediated upregulation of MnSOD.
Figure 5. Effect of LPS and PI3Kinase inhibitor on cellular ATP and nitrite levels, ROS production, and cell viability
A. B6-MCL cells were pretreated with wortmannin (100 ng/mL) for 30 min prior to adding LPS (100 ng/mL) or kept in media for 24 h. Supernatants were analyzed for TNF-α via ELISA. Data are presented as means of four independent experiments and error bars indicate the standard error of the mean. (p< 0.01). B. Cumulative nitrite levels in the supernatant were measured as described in Material and Methods. Data presented as mean ±SEM of % changes of untreated cells. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001. C. Mitochondrial reactive oxygen species (ROS) were measured using the probe MitoSox. As positive control cells were treated with antimycine A (AA) for 30 min prior to treatment with the MitoSox fluorescence probe. Cells were treated with LPS (100 ng/mL) in presence and absence of wortmannin (100 ng/mL). The data presented as fold change in mean intensity of MitoSOX fluorescence compared to PBS plus MitoSOX alone (negative control). LPS treatment led to a time dependent increase of mROS after 6 and 24h. Pretreatment of cells 30 min. prior to LPS stimulation led to a significant decrease in mROS production. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001. D. Murine macrophages (B6-MCL) were treated with LPS (100 ng/mL) for 6h in the presence or absence of wortmannin (100 ng/mL) or LY294002 (50 ng/mL) 30 min prior to LPS treatment. ATP concentrations were measured using the bioluminescence method. ATP levels increased in response to LPS. Pretreatment with wortmannin or LY294002 abrogated the LPS-mediated ATP upregulation. Data presented is the result of 3 independent experiments measured in triplicates. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001. E. Cell viability. 2×105 B6-MCL cells were plated in 96 well plate and treated with LPS (100 ng/mL) for 24h in the presence or absence of wortmannin (100 ng/mL) or LY294002 (50 ng/mL). Cell viability was assessed using MTT assay. Data presented as mean ±SEM of % changes of untreated cells. Using ANOVA Mann-Whitney U test p<0.05 was considered significant. * denotes a p-value <0.05 and ** expresses a p-value of <0.001.
Figure 6. LPS induced COX I and T-fam expression is MyD88/TRIF dependent
A. Wild type murine macrophages (WT) and MyD88−/− TRIF−/− cells were cultured side by side in the presence and absence of LPS (100 ng/mL) for 30 or 60 min. Total cell lysates were prepared and 20 µg of total protein was subjected to SDS-PAGE and Western blot analysis using phosphoepitope-specific antibodies for p85 and AKT (serine 473). Equal loading of protein was confirmed using total p85 and AKT antibodies. B. Wilt type murine macrophages and MyD88/TRIF−/− cells were cultured side by side in the presence and absence of LPS for 90 min, 3h and 6h. Total cell lysates were prepared and 20 µg of total protein was subjected to SDS-PAGE and Western blot analysis using antibody against T-fam and COX I. Equal loading of protein was confirmed using β-actin antibody. Wild type cells responded to LPS stimulation with a significant increase in T-fam starting at 90 min throughout 6h post treatment with LPS. Upregulation of COX I in wild type was seen starting at 3h after LPS stimulation. In contrast, MyD88/TRIF−/− cells did not respond to LPS with increased expression for T-fam and COX I. C. Bone marrow derived macrophages isolated from wild type (WT), TRIF or MYD88 deficient mice were cultured in parallel with LPS for 5, 30 and 60 min. Total cell lysates were prepared and 20 µg of total protein was subjected to SDS-PAGE and Western blot analysis using phosphoepitope-specific antibodies for p85 and AKT (serine 473). Equal loading was confirmed using total AKT and total p85 antibodies. LPS treatment evoked a weak and delayed AKT phosphorylation in TRIF−/− cells but MYD88 deficient cells did not show any response to LPS treatment. D. Bone marrow derived macrophages isolated from WT, TRIF or MYD88 deficient mice were either kept in media or treated with LPS for 3h. Twenty µg of total protein was subjected to SDS-PAGE and Western blot analysis using T-fam antibody. Equal loading was confirmed using β actin antibody. While LPS treatment led to an increase in T-fam expression in WT, neither TRIF−/− and MyD88−/− cells responded with an increased expression for T-Fam. E. Bone marrow derived macrophages isolated from WT, TRIF or MYD88 deficient mice were either kept in media or treated with LPS for 3h and 6h. Twenty µg of total protein was subjected to SDS-PAGE and Western blot analysis using a COX IV antibody. Equal loading was confirmed using total AKT antibody. While LPS treatment led to an increase in COX IV expression in WT after 6h, TRIF−/− and MyD88−/− cells did not show any expression of COX IV.
Figure 7. Critical role of AKT1 in LPS mediated COX expression
Bone marrow derived macrophages were isolated from WT, AKT1 or AKT2 deficient mice, cultured under equal conditions and treated with LPS for different time points as indicated. A. Detection of phospho p85 and AKT. Twenty µg of total protein was subjected to SDS-PAGE and Western blot analysis using phosphoepitope-specific antibodies for p85 and AKT (serine 473). Equal loadings were confirmed using either total p85 or total AKT antibodies. B. Detection of AKT isoforms. Immunoblot analysis of whole cell lysate performed using antibodies detecting AKT1 and AKT2. Equal loading was confirmed using β actin antibody. As shown AKT1−/− macrophages lack expression of AKT1 while AKT2−/− macrophages lack AKT2. C. Bone marrow derived macrophages isolated from WT, AKT1−/−, and AKT2−/− mice kept in media or treated with LPS for 6h. Immunoblot analysis of whole cell lysate performed using antibodies detecting COX I (upper panel) and COX IV (lower panel). Equal loading was confirmed using β antibody. Cells lacking AKT1 had severe impairment in COX I and COX IV expression at baseline as well as in response to LPS. Induction of COX I was unimpaired in response to LPS treatment in AKT2−/− macrophages. Furthermore, the basal level of COX IV was lower in these macrophages as compared to those of WT, but AKT2−/− macrophages responded to LPS with an enhanced expression for COX IV.
Similar articles
- Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells.
Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX, Song LH. Xia MZ, et al. J Pineal Res. 2012 Nov;53(4):325-34. doi: 10.1111/j.1600-079X.2012.01002.x. Epub 2012 Apr 27. J Pineal Res. 2012. PMID: 22537289 - Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling.
Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Piao W, et al. J Leukoc Biol. 2009 Oct;86(4):863-75. doi: 10.1189/jlb.0309189. Epub 2009 Aug 5. J Leukoc Biol. 2009. PMID: 19656901 Free PMC article. - The Asp299Gly polymorphism alters TLR4 signaling by interfering with recruitment of MyD88 and TRIF.
Figueroa L, Xiong Y, Song C, Piao W, Vogel SN, Medvedev AE. Figueroa L, et al. J Immunol. 2012 May 1;188(9):4506-15. doi: 10.4049/jimmunol.1200202. Epub 2012 Apr 2. J Immunol. 2012. PMID: 22474023 Free PMC article. - Functional role of Akt in macrophage-mediated innate immunity.
Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH, Lee SY, Cho JY. Lee YG, et al. Front Biosci (Landmark Ed). 2011 Jan 1;16(2):517-30. doi: 10.2741/3702. Front Biosci (Landmark Ed). 2011. PMID: 21196185 Review. - Uncovering the source of mitochondrial superoxide in pro-inflammatory macrophages: Insights from immunometabolism.
Casey AM, Murphy MP. Casey AM, et al. Biochim Biophys Acta Mol Basis Dis. 2022 Oct 1;1868(10):166481. doi: 10.1016/j.bbadis.2022.166481. Epub 2022 Jul 2. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 35792320 Free PMC article. Review.
Cited by
- Syringin from Tinospora crispa downregulates pro-inflammatory mediator production through MyD88-dependent pathways in lipopolysaccharide (LPS)-induced U937 macrophages.
Arshad L, Haque MA, Harikrishnan H, Ibrahim S, Jantan I. Arshad L, et al. Mol Biol Rep. 2024 Jul 11;51(1):789. doi: 10.1007/s11033-024-09722-z. Mol Biol Rep. 2024. PMID: 38990383 - Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K.
Troutman TD, Bazan JF, Pasare C. Troutman TD, et al. Cell Cycle. 2012 Oct 1;11(19):3559-67. doi: 10.4161/cc.21572. Epub 2012 Aug 16. Cell Cycle. 2012. PMID: 22895011 Free PMC article. Review. - Early Growth Response Gene-2 Is Essential for M1 and M2 Macrophage Activation and Plasticity by Modulation of the Transcription Factor CEBPβ.
Veremeyko T, Yung AWY, Anthony DC, Strekalova T, Ponomarev ED. Veremeyko T, et al. Front Immunol. 2018 Nov 1;9:2515. doi: 10.3389/fimmu.2018.02515. eCollection 2018. Front Immunol. 2018. PMID: 30443252 Free PMC article. - TLR4 increases the stemness and is highly expressed in relapsed human hepatocellular carcinoma.
Zhou S, Du R, Wang Z, Shen W, Gao R, Jiang S, Fang Y, Shi Y, Chang A, Liu L, Liu C, Li N, Xiang R. Zhou S, et al. Cancer Med. 2019 May;8(5):2325-2337. doi: 10.1002/cam4.2070. Epub 2019 Apr 8. Cancer Med. 2019. PMID: 30957973 Free PMC article. - Calcium/calmodulin-dependent protein kinase regulates the PINK1/Parkin and DJ-1 pathways of mitophagy during sepsis.
Zhang X, Yuan D, Sun Q, Xu L, Lee E, Lewis AJ, Zuckerbraun BS, Rosengart MR. Zhang X, et al. FASEB J. 2017 Oct;31(10):4382-4395. doi: 10.1096/fj.201601096RRR. Epub 2017 Jun 14. FASEB J. 2017. PMID: 28615325 Free PMC article.
References
- Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochemical pharmacology. 2006;72:1102–1113. - PubMed
- Rich P. Chemiosmotic coupling: The cost of living. Nature. 2003;421:583. - PubMed
- Levy RJ, Deutschman CS. Cytochrome c oxidase dysfunction in sepsis. Crit Care Med. 2007;35:S468–S475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK061707-01/DK/NIDDK NIH HHS/United States
- R01 DK61707/DK/NIDDK NIH HHS/United States
- R01 DK061707/DK/NIDDK NIH HHS/United States
- R01 DK056886/DK/NIDDK NIH HHS/United States
- R01 DK56886/DK/NIDDK NIH HHS/United States
- R01 DK056886-01/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous