TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis - PubMed (original) (raw)
TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis
Karel Otero et al. J Immunol. 2012.
Erratum in
- J Immunol. 2012 Jun 1;188(11):5802
Abstract
TREM2 is an immunoreceptor expressed on osteoclasts (OC) and microglia that transmits intracellular signals through the adaptor DAP12. Individuals with genetic mutations inactivating TREM2 or DAP12 develop the Nasu-Hakola disease (NHD) with cystic-like lesions of the bone and brain demyelination that lead to fractures and presenile dementia. The mechanisms of this disease are poorly understood. In this study, we report that TREM2-deficient mice have an osteopenic phenotype reminiscent of NHD. In vitro, lack of TREM2 impairs proliferation and β-catenin activation in osteoclast precursors (OcP) in response to M-CSF. This defect results in accelerated differentiation of OcP into mature OC. Corroborating the importance of a balanced proliferation and differentiation of OcP for bone homeostasis, we show that conditional deletion of β-catenin in OcP also results in reduced OcP proliferation and accelerated osteoclastogenesis in vitro as well as osteopenia in vivo. These results reveal that TREM2 regulates the rate of osteoclastogenesis and provide a mechanism for the bone pathology in NHD.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures
Figure 1
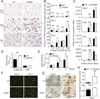
TREM2 deficiency results in an osteopenic phenotype. (A) Microcomputed tomography (µCT) analysis of the femurs of WT and TREM2−/− deficient mice. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 8 weeks. (B) osteoclastic and (C) osteoblastic parameters were obtained from bone morphometric analysis of mice at the age of 8 weeks. Graphs show mean ± SEM, N=5. *, P < 0.05; **, P < 0.01.
Figure 2
TREM2-deficient osteoclast precursors exhibit accelerated osteoclastogenesis. OcP generated from WT and TREM2−/− mice were cultured in vitro with 10 ng/ml M-CSF and 100 ng/ml RANKL to generate multinuclear osteoclasts. (A and B) Development of osteoclasts was monitored at different timepoints by TRAP staining. (A) Representative TRAP-stained images of the cultures. (B) The number of TRAP-positive cells containing three or more nuclei was scored (total). In addition, the nuclei in each osteoclast was enumerated as follows: 3–10; 6–10 and >10 nuclei per TRAP-positive cell. (C) Effect of TREM2 deficiency on the expression of the osteoclastic differentiation markers Nfatc1 (encoding NFATc1), Acp5 (encoding TRAP), Ctsk (encoding cathepsin K), and Calcr (encoding calcitonin receptor) measured by Q-PCR. (D) Quantification of TRAP activity in WT and TREM2−/− OcP and OC obtained by culturing OcP for 3 days with M-CSF and RANKL. (E) Apoptosis of osteoclast cultures as determined by quantification of DNA fragments by ELISA. (F, G and H) Osteoclast differentiation induced on bone slices. (F) After 4 and 7 days of differentiation cultures were fixed and stained with Phalloidin-FITC to visualize the osteoclast actin rings. (G) Bone resorption pits were revealed by lectin staining of the bone slices (brown reaction product). (H) Supernatants of the cultures were collected at day 4 and 6 of culture and bone resorption was assessed by measuring the degradation products of the C-terminal telopeptides of Type I collagen by ELISA. *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
Figure 3
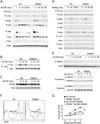
Impaired M-CSF-induced β-catenin expression and cell proliferation of TREM2-deficient osteoclast precursors. (A–E) OcP generated from WT and TREM2−/− mice were starved from M-CSF for 4 h and then exposed to 50 ng/ml M-CSF (A, B, C and E) or were starved from M-CSF and serum for 4 h and then exposed to 100 ng/ml RANKL (D). After the indicated times total cell lysates were prepared and subjected to immunoprecipitation and/or immunoblotting analysis using antibodies to the indicated proteins. Actin served as loading control for total lysates. (A) M-CSF-induced phosphorylation of various signaling mediators. (B) TREM2-dependent activation of Syk by M-CSF. After the indicated times of M-CSF stimulation total cell lysates were prepared and immunoprecipitated (IP) with anti-Syk Ab. Immunoblots were performed with anti-phosphotyrosine Ab to detect activated Syk. To control for protein loading, the membranes were reprobed for total Syk. (C) TREM2-dependent activation of Pyk2 by M-CSF. Immunoblots of total cell lysates were performed with anti-phospho-Pyk2 and anti-Pyk2. (D) RANKL-induced phosphorylation of various signaling mediators. (E) M-CSF-induced β-catenin expression and its nuclear translocation is dependent of TREM2. OcP were cultured in the absence of M-CSF for 4 h and then restimulated with M-CSF (100 ng/ml) and the expression of β-catenin in total lysates, cytoplasmic and nuclear cell fractions was analyzed by inmunoblotting. Actin, GAPDH and lamin-B served as loading controls for total cell lysates, cytoplasmic and nuclear cell fractions respectively. (F) Defect of M-CSF-induced proliferation in TREM2-deficient OcP. Proliferation of WT and TREM2−/− OcP was measured 12 h after incubation with BrdU in the presence of 100 ng/ml M-CSF. (G) Time course of changes in cell growth in OcP cultured in the presence of osteoclastogenic cytokines. OcP were cultured with 100 ng/ml M-CSF with or without 100 ng/ml RANKL. After culturing for the indicating periods, cell growth was measured by the MTT assay and expressed as the increase in OD560nm -background OD670nm relative to day 0. *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
Figure 4
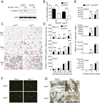
β-catenin-deficient osteoclast precursors proliferate less to M-CSF and exhibit accelerated osteoclastogenesis. (A) Immunoblot analysis of β-catenin and actin (loading control) in OcP total protein lysates from control LysM-Cre+/+ β-catenin+/+ (β-cat+/+) and LysM-Cre+/+ β-cateninfl/fl (β-catΔ̃Δ mice. (B) Defect of M-CSF-induced proliferation in β-catenin-deficient OcP. Proliferation of β-cat+/+ and β-catΔ̃Δ OcP was measured 12 h after incubation with BrdU in the presence of 100 ng/ml M-CSF. (C and D) OcP from β-cat+/+ and β-catΔ̃Δ mice were cultured with 10 ng/ml M-CSF and 100 ng/ml RANKL to generate multinuclear osteoclasts. Development of osteoclasts was monitored at different timepoints by TRAP staining. (C) Representative images of TRAP-stained cultures. (D) The number of TRAP-positive cells containing three or more nuclei was scored (total). In addition, the nuclei in each osteoclast was enumerated as follows: 3–10; 6–10 and >10 nuclei per TRAP-positive cell. (E) Effect of β-catenin deficiency on the expression of the osteoclastic differentiation markers Nfatc1 (encoding NFATc1), Acp5 (encoding TRAP), Ctsk (encoding cathepsin K), and Calcr (encoding calcitonin receptor) by Q-PCR. (F and G) Osteoclast differentiation induced on bone slices. (F) After 4 and 7 days of differentiation cultures were fixed and stained with Phalloidin-FITC to visualize the osteoclast actin rings. (G) Bone resorption pits were revealed by lectin staining of the bone slices (brown reaction product). *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
Figure 5
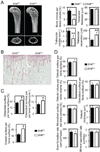
Mice deficient for β-catenin in osteoclast precursors are osteopenic. (A) Microcomputed tomography (µCT) analysis of the femurs of LysM-Cre+/+ β-catenin+/+ (β-cat+/+) and LysM-Cre+/+ β-cateninfl/fl (β-catΔ̃Δ mice. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 6 weeks. (B–D) Histological analysis of the tibiae. (B) Photographs represent TRAP and hematoxilin staining. (C) Osteoclastic and (D) osteoblastic parameters were obtained from bone morphometric analysis of mice at the age of 6 weeks. Graphs show mean ± SEM, N=5. *, P < 0.05; **, P < 0.01.
Figure 6
TREM-2 and β -catenin function together to maintain osteoclast numbers and bone homeostasis. We generated mice with the genotype LysM-Cre+/+ with the allelic combinations β-catenin+/+ Trem2+/+ (Control), β-cateninfl/+ Trem2+/+ (βcatfl/+), β-catenin+/+ Trem2−/+ (Trem2−/+), and β-cateninfl/+ Trem2−/+(βcatfl/+Trem2−/+). Femurs were analyzed by µCT. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 8 weeks. Graphs show mean ± SEM, N=5. **, P < 0.01.
Similar articles
- TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function.
Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC. Humphrey MB, et al. J Bone Miner Res. 2006 Feb;21(2):237-45. doi: 10.1359/JBMR.051016. Epub 2005 Oct 20. J Bone Miner Res. 2006. PMID: 16418779 - DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features.
Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. Paloneva J, et al. J Exp Med. 2003 Aug 18;198(4):669-75. doi: 10.1084/jem.20030027. J Exp Med. 2003. PMID: 12925681 Free PMC article. - TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway.
Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, Xu H, Bu G. Zheng H, et al. J Neurosci. 2017 Feb 15;37(7):1772-1784. doi: 10.1523/JNEUROSCI.2459-16.2017. Epub 2017 Jan 11. J Neurosci. 2017. PMID: 28077724 Free PMC article. - The enigmatic function of TREM-2 in osteoclastogenesis.
Colonna M, Turnbull I, Klesney-Tait J. Colonna M, et al. Adv Exp Med Biol. 2007;602:97-105. doi: 10.1007/978-0-387-72009-8_13. Adv Exp Med Biol. 2007. PMID: 17966394 Review. - Non-pathological roles of microglial TREM2/DAP12: TREM2/DAP12 regulates the physiological functions of microglia from development to aging.
Konishi H, Kiyama H. Konishi H, et al. Neurochem Int. 2020 Dec;141:104878. doi: 10.1016/j.neuint.2020.104878. Epub 2020 Oct 10. Neurochem Int. 2020. PMID: 33049336 Review.
Cited by
- Osteoimmunology: Major and Costimulatory Pathway Expression Associated with Chronic Inflammatory Induced Bone Loss.
Crotti TN, Dharmapatni AA, Alias E, Haynes DR. Crotti TN, et al. J Immunol Res. 2015;2015:281287. doi: 10.1155/2015/281287. Epub 2015 May 3. J Immunol Res. 2015. PMID: 26064999 Free PMC article. Review. - RNA-Binding Proteins as Novel Effectors in Osteoblasts and Osteoclasts: A Systems Biology Approach to Dissect the Transcriptional Landscape.
Meshcheryakova A, Bohdan S, Zimmermann P, Jaritz M, Pietschmann P, Mechtcheriakova D. Meshcheryakova A, et al. Int J Mol Sci. 2024 Sep 27;25(19):10417. doi: 10.3390/ijms251910417. Int J Mol Sci. 2024. PMID: 39408753 Free PMC article. - Function and mechanism of TREM2 in bacterial infection.
Wu Z, Yang S, Fang X, Shu Q, Chen Q. Wu Z, et al. PLoS Pathog. 2024 Jan 18;20(1):e1011895. doi: 10.1371/journal.ppat.1011895. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38236825 Free PMC article. Review. - CathepsinKCre mediated deletion of βcatenin results in dramatic loss of bone mass by targeting both osteoclasts and osteoblastic cells.
Ruiz P, Martin-Millan M, Gonzalez-Martin MC, Almeida M, González-Macias J, Ros MA. Ruiz P, et al. Sci Rep. 2016 Nov 2;6:36201. doi: 10.1038/srep36201. Sci Rep. 2016. PMID: 27804995 Free PMC article. - Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer's disease.
McQuade A, Kang YJ, Hasselmann J, Jairaman A, Sotelo A, Coburn M, Shabestari SK, Chadarevian JP, Fote G, Tu CH, Danhash E, Silva J, Martinez E, Cotman C, Prieto GA, Thompson LM, Steffan JS, Smith I, Davtyan H, Cahalan M, Cho H, Blurton-Jones M. McQuade A, et al. Nat Commun. 2020 Oct 23;11(1):5370. doi: 10.1038/s41467-020-19227-5. Nat Commun. 2020. PMID: 33097708 Free PMC article.
References
- Leibbrandt A, Penninger JM. RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv. Exp. Med. Biol. 2009;649:100–113. - PubMed
- Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, Delaisse JM, Takayanagi H, Lorenzo J, Colonna M, Farndale RW, Choi Y, Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 2011;121:3505–3516. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AR046523/AR/NIAMS NIH HHS/United States
- 5T32AI7163-32/AI/NIAID NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- R01 AR057037/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials