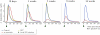Impaired neurotransmission caused by overexpression of α-synuclein in nigral dopamine neurons - PubMed (original) (raw)
Impaired neurotransmission caused by overexpression of α-synuclein in nigral dopamine neurons
Martin Lundblad et al. Proc Natl Acad Sci U S A. 2012.
Abstract
We used in vivo amperometry to monitor changes in synaptic dopamine (DA) release in the striatum induced by overexpression of human wild-type α-synuclein in nigral DA neurons, induced by injection of an adeno-associated virus type 6 (AAV6)-α-synuclein vector unilaterally into the substantia nigra in adult rats. Impairments in DA release evolved in parallel with the development of degenerative changes in the nigrostriatal axons and terminals. The earliest change, seen 10 d after vector injection, was a marked, ≈50%, reduction in DA reuptake, consistent with an early dysfunction of the DA transporter that developed before any overt signs of axonal damage. At 3 wk, when the first signs of axonal damage were observed, the amount of DA released after a KCl pulse was reduced by 70-80%, and peak DA concentration was delayed, indicating an impaired release mechanism. At later time points, 8-16 wk, overall striatal innervation density was reduced by 60-80% and accompanied by abundant signs of axonal damage in the form of α-synuclein aggregates, axonal swellings, and dystrophic axonal profiles. At this stage DA release and reuptake were profoundly reduced, by 80-90%. The early changes in synaptic DA release induced by overexpression of human α-synuclein support the idea that early predegenerative changes in the handling of DA may initiate, and drive, a progressive degenerative process that hits the axons and terminals first. Synaptic dysfunction and axonopathy would thus be the hallmark of presymptomatic and early-stage Parkinson disease, followed by neuronal degeneration and cell loss, characteristic of more advanced stages of the disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
(A) Coronal sections through the forebrain and midbrain, stained with a human-specific α-synuclein antibody, from a rat that received a unilateral 3-μL injection of the AAV–α-synuclein vector just above the substantia nigra (asterisk) 10 d erlier. Human WT α-synuclein derived from the vector is expressed in the DA neurons of the substantia nigra and distributed throughout the ipsilateral caudate-putamen. Inset: Electrode/micropipette assembly used for recording; the vertical lines mark the bilateral recording sites. (B) The four measurement parameters—peak amplitude, linear rise rate, reuptake rate, and AUC—were calculated from the DA release curve, as indicated.
Fig. 2.
Traces showing the change in extracellular DA concentration recorded simultaneously from the noninjected control side (blue) and the AAV–α-synuclein injected side (red), elicited by the KCl pulse, at four time points after vector injection. Note the pronounced broadening of the DA release curve seen already at the 10-d time point and the progressive reduction in the peak amplitude. Yellow traces show DA release recorded from animals injected with the AAV-GFP control vector.
Fig. 3.
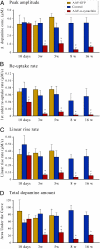
Changes in (A) peak amplitude, (B) reuptake rate, (C) linear rise rate, and (D) AUC, recorded simultaneously at the five time points from the AAV–α-synuclein injected side (red bars) and the noninjected control side (blue bars). A significant reduction in the DA reuptake rate is seen already at 10 d. Yellow bars show recordings from separate groups of animals injected with the AAV-GFP control vector. *P < 0.05; one-way ANOVA followed by Student t test.
Fig. 4.
Changes in (A) peak amplitude, (B) reuptake rate, (C) linear rise rate, and (D) AUC, induced by the WPRE-lacking AAV–α-synuclein vector (n = 3). As in Fig. 3, the recordings were made simultaneously from the AAV–α-synuclein injected side (red bars) and the noninjected control side (blue bars). (E) Actual traces from the two sides in one of the recorded rats. *P < 0.05; Student t test.
Fig. 5.
Changes in DA reuptake were studied, at 10 d after AAV–α-synuclein injection, after local injection of DA. (A) A pulse of DA was injected adjacent to the electrode tip, and the extracellular concentration of DA was monitored over the subsequent 200 s on the AAV–α-synuclein injected side (red) and the noninjected control side (blue). As shown in B, the elimination of extracellular DA was considerably slower on the α-synuclein–overexpressing side (red bar; n = 4), and the DA reuptake rate, calculated as indicated by the dashed lines in A, was reduced by approximately 60% compared with the contralateral intact striatum (blue bar; n = 4). *P < 0.05; one-way ANOVA followed by Student t test.
Fig. 6.
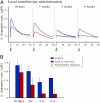
(A) Traces showing the change in extracellular DA concentration on the noninjected control side (blue) and the AAV–α-synuclein injected side (red), elicited by local application of the DA reuptake blocker nomifensine, at four time points after vector injection. (B) From 3 wk, onward, there was a significant reduction in the peak amplitude on the AAV–α-synuclein injected side (red bars), compared with the simultaneous recording on the noninjected control side (blue bars), signifying an impaired DA release machinery (n = 4 per group). *P < 0.05; one-way ANOVA followed by Student t test.
Fig. 7.
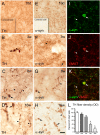
Changes in the striatal DA terminal network induced by overexpression of human WT α-synuclein, as observed in sections stained for TH (A–D) and human α-synuclein (E–H). The first pathological changes, observed as scattered swollen dystrophic axonal segments, appeared at 3 wk after vector injection (B and F). At 8 wk (C and G) and 16 wk (D and H) the overall density of TH- and α-synuclein–positive axons and terminal were markedly reduced, and many of the remaining terminals showed signs of damage, expressed as TH- and α-synuclein–containing axonal swellings (arrowheads), some of them with the appearance of large end-bulbs (arrows). Some of these α-synuclein–positive axonal swellings stained positively for VMAT-2 (I–K). (L) Progressive decline in striatal TH immunorectivity on the vector-injected side, as measured by densitometry (n = 4–6 per group). *P < 0.05; one-way ANOVA followed by Student t test. (Scale bar, 10 μm.)
Fig. 8.
Sections stained for DAT (A–C) and VMAT-2 (D–F) showed that the expression of DAT and the vesicular transporter, in individual striatal DA terminals, was maintained at normal levels at both 10 d (A and D) and 3 wk (B and E) after AAV_–_α-synuclein injection. (F) At 8 wk, the swollen, dystrophic axons and terminals stained positively for VMAT-2. (C) DAT, by contrast, was expressed at seemingly normal levels in the remaining terminals but not in axons with swollen, dystrophic profiles. (Scale bar, 10 μm.)
Fig. 9.
The changes that develop in the striatal dopaminergic innervation over time make it possible to define distinct stages of damage and impairment that correspond to the presymptomatic, early symptomatic, and advanced stages of the human disease. The presymptomatic stage, which develops within the first 3 wk after AAV_–_α-synuclein injection, is characterized by impaired transmitter release and reuptake at the synaptic level, and early signs of axonal damage, but without any major cell loss. The first signs of behavioral impairments, representing an early symptomatic stage, are seen at 5 wk after vector injection. At this stage neurodegeneration is well under way, accompanied by a partial loss of the striatal dopaminergic innervation, more abundant signs of axonal damage, and a reduction in striatal DA levels. The advanced stage, seen at 8–16 wk, when degeneration of the nigral DA neurons is complete, is characterized by severe loss of TH-positive terminals in the striatum, accompanied by significant impairment in motor behavior. Remaining axons and terminals in the striatum show signs of ongoing pathology, including large α-synuclein-, TH-, and VMAT-2_–_containing dystrophic axonal profiles. Our amperometry data suggest that this remaining innervation is dysfunctional but that the severe reduction of DA reuptake, which far exceeds the magnitude of loss of TH-positive terminals, will help to maintain DA neurotransmission at a functional level and thus delay the appearance of more profound motor deficits.
Similar articles
- Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons.
Decressac M, Mattsson B, Lundblad M, Weikop P, Björklund A. Decressac M, et al. Neurobiol Dis. 2012 Mar;45(3):939-53. doi: 10.1016/j.nbd.2011.12.013. Epub 2011 Dec 11. Neurobiol Dis. 2012. PMID: 22182688 - Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system.
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Björklund A. Kirik D, et al. J Neurosci. 2002 Apr 1;22(7):2780-91. doi: 10.1523/JNEUROSCI.22-07-02780.2002. J Neurosci. 2002. PMID: 11923443 Free PMC article. - Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity.
Gaugler MN, Genc O, Bobela W, Mohanna S, Ardah MT, El-Agnaf OM, Cantoni M, Bensadoun JC, Schneggenburger R, Knott GW, Aebischer P, Schneider BL. Gaugler MN, et al. Acta Neuropathol. 2012 May;123(5):653-69. doi: 10.1007/s00401-012-0963-y. Epub 2012 Feb 24. Acta Neuropathol. 2012. PMID: 22361813 - Viral vector-mediated overexpression of α-synuclein as a progressive model of Parkinson's disease.
Ulusoy A, Decressac M, Kirik D, Björklund A. Ulusoy A, et al. Prog Brain Res. 2010;184:89-111. doi: 10.1016/S0079-6123(10)84005-1. Prog Brain Res. 2010. PMID: 20887871 Review. - Synaptic dysfunction in Parkinson's disease.
Picconi B, Piccoli G, Calabresi P. Picconi B, et al. Adv Exp Med Biol. 2012;970:553-72. doi: 10.1007/978-3-7091-0932-8_24. Adv Exp Med Biol. 2012. PMID: 22351072 Review.
Cited by
- In Parkinson's patient-derived dopamine neurons, the triplication of α-synuclein locus induces distinctive firing pattern by impeding D2 receptor autoinhibition.
Lin M, Mackie PM, Shaerzadeh F, Gamble-George J, Miller DR, Martyniuk CJ, Khoshbouei H. Lin M, et al. Acta Neuropathol Commun. 2021 Jun 7;9(1):107. doi: 10.1186/s40478-021-01203-9. Acta Neuropathol Commun. 2021. PMID: 34099060 Free PMC article. - Transcriptomic profiling of early synucleinopathy in rats induced with preformed fibrils.
Patterson JR, Kochmanski J, Stoll AC, Kubik M, Kemp CJ, Duffy MF, Thompson K, Howe JW, Cole-Strauss A, Kuhn NC, Miller KM, Nelson S, Onyekpe CU, Beck JS, Counts SE, Bernstein AI, Steece-Collier K, Luk KC, Sortwell CE. Patterson JR, et al. NPJ Parkinsons Dis. 2024 Jan 3;10(1):7. doi: 10.1038/s41531-023-00620-y. NPJ Parkinsons Dis. 2024. PMID: 38172128 Free PMC article. - A Proposed Roadmap for Parkinson's Disease Proof of Concept Clinical Trials Investigating Compounds Targeting Alpha-Synuclein.
Merchant KM, Cedarbaum JM, Brundin P, Dave KD, Eberling J, Espay AJ, Hutten SJ, Javidnia M, Luthman J, Maetzler W, Menalled L, Reimer AN, Stoessl AJ, Weiner DM; The Michael J. Fox Foundation Alpha Synuclein Clinical Path Working Group. Merchant KM, et al. J Parkinsons Dis. 2019;9(1):31-61. doi: 10.3233/JPD-181471. J Parkinsons Dis. 2019. PMID: 30400107 Free PMC article. - Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects.
Khodr CE, Becerra A, Han Y, Bohn MC. Khodr CE, et al. Brain Res. 2014 Mar 6;1550:47-60. doi: 10.1016/j.brainres.2014.01.010. Epub 2014 Jan 21. Brain Res. 2014. PMID: 24463035 Free PMC article. - Chronic treatment with novel small molecule Hsp90 inhibitors rescues striatal dopamine levels but not α-synuclein-induced neuronal cell loss.
McFarland NR, Dimant H, Kibuuka L, Ebrahimi-Fakhari D, Desjardins CA, Danzer KM, Danzer M, Fan Z, Schwarzschild MA, Hirst W, McLean PJ. McFarland NR, et al. PLoS One. 2014 Jan 20;9(1):e86048. doi: 10.1371/journal.pone.0086048. eCollection 2014. PLoS One. 2014. PMID: 24465863 Free PMC article.
References
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. - PubMed
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. - PubMed
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. - PubMed
- Ma SY, Röyttä M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J Neurol Sci. 1997;151:83–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources