Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7 - PubMed (original) (raw)
Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7
Kathryn M Taylor et al. Sci Signal. 2012.
Abstract
The transition element zinc, which has recently been identified as an intracellular second messenger, has been implicated in various signaling pathways, including those leading to cell proliferation. Zinc channels of the ZIP (ZRT1- and IRT1-like protein) family [also known as solute carrier family 39A (SLC39A)] transiently increase the cytosolic free zinc (Zn(2+)) concentration in response to extracellular signals. We show that phosphorylation of evolutionarily conserved residues in endoplasmic reticulum zinc channel ZIP7 is associated with the gated release of Zn(2+) from intracellular stores, leading to activation of tyrosine kinases and the phosphorylation of AKT and extracellular signal-regulated kinases 1 and 2. Through pharmacological manipulation, proximity ligation assay, and mutagenesis, we identified protein kinase CK2 as the kinase responsible for ZIP7 activation. Together, the present results show that transition element channels in eukaryotes can be activated posttranslationally by phosphorylation, as part of a cell signaling cascade. Our study links the regulated release of zinc from intracellular stores to phosphorylation of kinases involved in proliferative responses and cell migration, suggesting a functional role for ZIP7 and zinc signals in these events. The connection with proliferation and migration, as well as the activation of ZIP7 by CK2, a kinase that is antiapoptotic and promotes cell division, suggests that ZIP7 may provide a target for anticancer drug development.
Figures
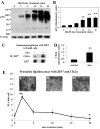
Figure 1. Zinc release activates tyrosine kinases downstream of CK2 binding ZIP7
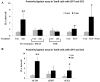
(A-B) TamR cells were treated with 20 μM zinc plus sodium pyrithione and lysates probed by Western analysis with a phosphotyrosine (pTyr) antibody (A). pTyr, quantified by densitometry on blots from three independent experiments was normalized to β-actin and displayed as mean ± SD with significant (P ≤ 0.001) changes over time 0 indicated by ** (B). (C-D) TamR cells, treated with zinc for 5 minutes, were immunoprecipitated with antibody directed against ZIP7. Western Blot was probed with antibodies directed against CK2α (C) or ZIP7 (as loading control); mean values ± SD calculated from N=3 experiments and normalized to ZIP7 (D) showed a significant change (P ≤ 0.001 indicated by **) in the association of CK2α and ZIP7. (E) Proximity ligation assay was performed with anti-ZIP7 and anti-CK2α antibodies in TamR cells treated with zinc. Fluorescent dots in inserts demonstrate ZIP7 and CK2α in close proximity (<40nm). Complete time course and antibody-free controls shown in fig. S2. Pooled results as 25 stacks taken 0.3μm apart from at least 6 representative fields of view in 3 experiments are expressed as mean dots/cell ± SD. Significant changes compared to time 0 are indicated by * (P ≤ 0.05) and ** (P ≤ 0.001). Scale bar=10μm.
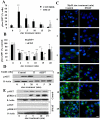
Figure 2. CK2 inhibition decreases ZIP7-dependent zinc transport
(A) FACS analysis of TamR cells treated with zinc plus sodium pyrithione and probed with antibodies directed against pSer and ZIP7. The increased percentage of cells with activated pSer abundance was recorded as maximum at 2 minutes after zinc treatment (** P ≤ 0.001) as a function of ZIP7 and was abolished by DMAT pretreatment. (B) Parallel FACS analysis demonstrated that CK2 or ZIP7 siRNA attenuated the increase in pSer as a function of ZIP7 by over 50%. Results displayed in panel B represent cells with increased pSer expressed as a percentage of control cells with the mean ± SD calculated from n=3 experiments. (C) TamR cells (control), loaded with Fluozin-3 showed increased green fluorescence after 10 minutes of zinc treatment; pre-treatment with CK2 inhibitor DMAT largely prevented this increase. Nuclei were counterstained blue with DAPI. Scale bar=10μm. (D-E) ZIP7 siRNA significantly decreased pAKT at 5 minutes (P ≤ 0.05, fig. S2) (D) and the CK2 inhibitor DMAT reduced the significant increase in pAKT at 5, 10 and 15 mins (P ≤ 0.05, Fig. S2) and pERK1/2 at 5 min (P ≤ 0.001, Fig. S2) and 10 and 15 mins (P ≤ 0.05, Fig. S2) to undetectable levels (E); representative of N=3 experiments. See fig. S2 for quantification of blots D-E.
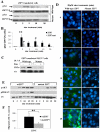
Figure 3. Mutation of ZIP7 (S275A:S276A) prevents its association with CK2 and zinc release
(A) MCF-7 cells transfected with wild type or mutant ZIP7 were treated with zinc and analysed for pSer by Western blot. There was significant activation of pSer in cells expressing wild-type ZIP7 at 2 and 5 mins (P ≤ 0.001 and P ≤ 0.05, respectively, see Fig. S4) whereas this was absent in cells expressing ZIP7 mutant. Representative of n=3 blots. (B) Parallel FACS experiments determined the extent of activated pSer in ZIP7 positive cells in N=3 experiments and expressed as mean ± SD. Cells expressing wild-type ZIP7 showed increased activation of pSer throughout compared to time 0 (indicated by * (P ≤ 0.05) and ** (P ≤ 0.001), especially at 2 minutes, in contrast to cells expressing mutant ZIP7 which showed a small 10% increase only at the 2 minute time point. (C) Recombinant ZIP7 proteins immunoprecipitated with V5 antibody after 5 minutes of zinc treatment and probed for CK2α. Cells transfected with wild-type ZIP7 showed a significant increase in CK2α when treated with zinc (P ≤ 0.05, see Fig S4). Representative of N=3 experiments.(D) Cells expressing mutant ZIP7 did not show increased green Fluozin-3 fluorescence after zinc treatment, indicative of no cytosolic Zn2+ release. Nuclei were counterstained blue with DAPI. Representative of N =3 experiments Scale bar=10μm. (E) Cells expressing mutant ZIP7, in contrast to wild-type ZIP7, did not show a significant ERK1/2 phosphorylation in response to zinc treatment (P ≤ 0.05, Fig. S4) and showed a significantly decreased phosphorylation of AKT (see Fig.S4). Representative of N=3 experiments (F) Mutant ZIP7 transfected cells showed significantly decreased migratory potential over 48 hours in the presence of zinc compared to cells transfected with wild-type ZIP7 (P<0.001 using an independent t-test, indicated by **) N=3 experiments. See Fig. S4 for quantification of Western blots in this figure.
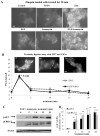
Figure 4. Zinc release from stores can be stimulated without exogenous zinc treatment
(A) TamR cells were loaded with zinquin, exposed to different stimuli, and imaged 20 minutes later to assess intracellular zinc. Fluorescence with EGF+ionomycin was comparable to that with zinc plus sodium pyrithione, indicating that EGF+ionomycin stimulated zinc release into the cytosol in the absence of extracellular zinc. Representative of N= 3 experiments. Scale bar=10μm. (B) Proximity ligation assay performed in TamR cells with anti-ZIP7 and anti-CK2α antibodies demonstrates that EGF + ionomycin generate a transient association between ZIP7 and CK2, with a maximal increase compared to time 0 (* P ≤ 0.05) at 2 minutes. Overlaid with effects of zinc treatment to aid comparison. Fluorescent dots in inserts demonstrate ZIP7 and CK2α in close proximity (<40nm). Complete time course and validation of CK2 antibody shown in Fig. S5. Pooled results from over 3 representative fields from each of N=3 experiments are expressed as mean ± SD dots/cell. Scale bar=10μm. (C-D) Western blot analysis of pAKT and pERK1/2 confirmed that treatment with EGF and ionomycin showed phosphorylation consistent with cytosolic Zn2+ release. Densitometry of n=3 blots normalized to β-actin demonstrate a significant increase compared to time 0 (** P ≤ 0.001), results are given as mean ± SD.
Figure 5. CK2 is essential for ZIP7 activation
(A-B) Duolink proximity assay performed with rabbit anti-ZIP7 and mouse anti-CK2 antibodies to assess the association of these molecules within TamR cells under various conditions. Pooled results from at least 3 representative fields of view from each of N=3 experiments are expressed as mean ± SD dots/cell. Significant changes compared to control cells with no treatment are indicated by * (P ≤ 0.05) and ** (P ≤ 0.001). (A) TamR cells stimulated with: 20μM zinc with 10μM zinc ionophore (sodium pyrithione), EGF (10ng/ml), or EGF (10ng/ml) and ionomycin (500nM). generated a significant increase in the association of ZIP7 with CK2α. The zinc-induced association was abolished by pre-treatment with the CK2 inhibitors DMAT or TBB. (B) Transfection of TamR cells for 3 days with siRNA directed toward either ZIP7 or CK2 suppressed the zinc stimulated increase in association observed between these molecules.
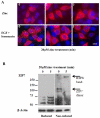
Figure 6. Intracellular location and quaternary structure of ZIP7 during zinc treatment
(A) Imaging of recombinant wild-type ZIP7 in TamR cells after treatment with zinc or EGF + ionomycin in permeabilized cells probed with V5 antibody which was conjugated to Alexa-Fluor 594 (red). The nuclei were counterstained blue with DAPI. Representative of N= 3 experiments. Scale bar=10μm. (B) TamR cells treated with zinc were probed for ZIP7 under reducing or non-reducing conditions. Under non-reducing conditions the ZIP7 band approximating 50kDa, increased to100kDa, a size consistent with its dimerization. Under reducing conditions, zinc treatment increased the two bands between 50-75kDa that were replaced under non-reducing conditions with a high molecular mass band greater than 250kDa. The lower bands indicative of cellular processing were evident in both reducing and non-reducing conditions. Representative of N= 3 blots.
Figure 7. Schematic showing the temporal relationship of CK2 association with ZIP7 and zinc release
Schematic illustrating the time course of the effect of zinc treatment on phosphorylation of ZIP7 by CK2 and the subsequent zinc release from the endoplasmic reticulum. The ticks represent relative abundance where 3 ticks represents maximum.
Similar articles
- Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling.
Nimmanon T, Ziliotto S, Morris S, Flanagan L, Taylor KM. Nimmanon T, et al. Metallomics. 2017 May 24;9(5):471-481. doi: 10.1039/c6mt00286b. Metallomics. 2017. PMID: 28205653 Free PMC article. - The Zinc Transporter SLC39A7 (ZIP7) Is Essential for Regulation of Cytosolic Zinc Levels.
Woodruff G, Bouwkamp CG, de Vrij FM, Lovenberg T, Bonaventure P, Kushner SA, Harrington AW. Woodruff G, et al. Mol Pharmacol. 2018 Sep;94(3):1092-1100. doi: 10.1124/mol.118.112557. Epub 2018 Jul 6. Mol Pharmacol. 2018. PMID: 29980658 - Hyperglycemia-Induced Changes in ZIP7 and ZnT7 Expression Cause Zn2+ Release From the Sarco(endo)plasmic Reticulum and Mediate ER Stress in the Heart.
Tuncay E, Bitirim VC, Durak A, Carrat GRJ, Taylor KM, Rutter GA, Turan B. Tuncay E, et al. Diabetes. 2017 May;66(5):1346-1358. doi: 10.2337/db16-1099. Epub 2017 Feb 23. Diabetes. 2017. PMID: 28232492 - Why and how to investigate the role of protein phosphorylation in ZIP and ZnT zinc transporter activity and regulation.
Thingholm TE, Rönnstrand L, Rosenberg PA. Thingholm TE, et al. Cell Mol Life Sci. 2020 Aug;77(16):3085-3102. doi: 10.1007/s00018-020-03473-3. Epub 2020 Feb 19. Cell Mol Life Sci. 2020. PMID: 32076742 Free PMC article. Review. - Targeting the Zinc Transporter ZIP7 in the Treatment of Insulin Resistance and Type 2 Diabetes.
Adulcikas J, Sonda S, Norouzi S, Sohal SS, Myers S. Adulcikas J, et al. Nutrients. 2019 Feb 15;11(2):408. doi: 10.3390/nu11020408. Nutrients. 2019. PMID: 30781350 Free PMC article. Review.
Cited by
- Zinc homeostasis and redox alterations in obesity.
Franco C, Canzoniero LMT. Franco C, et al. Front Endocrinol (Lausanne). 2024 Jan 8;14:1273177. doi: 10.3389/fendo.2023.1273177. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38260166 Free PMC article. Review. - Analysis of the prognostic significance of solute carrier (SLC) family 39 genes in breast cancer.
Liu L, Yang J, Wang C. Liu L, et al. Biosci Rep. 2020 Aug 28;40(8):BSR20200764. doi: 10.1042/BSR20200764. Biosci Rep. 2020. PMID: 32744318 Free PMC article. - Down-regulation of the islet-specific zinc transporter-8 (ZnT8) protects human insulinoma cells against inflammatory stress.
Merriman C, Fu D. Merriman C, et al. J Biol Chem. 2019 Nov 8;294(45):16992-17006. doi: 10.1074/jbc.RA119.010937. Epub 2019 Oct 7. J Biol Chem. 2019. PMID: 31591269 Free PMC article. - The biology of zinc transport in mammary epithelial cells: implications for mammary gland development, lactation, and involution.
McCormick NH, Hennigar SR, Kiselyov K, Kelleher SL. McCormick NH, et al. J Mammary Gland Biol Neoplasia. 2014 Mar;19(1):59-71. doi: 10.1007/s10911-013-9314-4. Epub 2013 Dec 15. J Mammary Gland Biol Neoplasia. 2014. PMID: 24338187 Review. - SLC39A7, regulated by miR-139-5p, induces cell proliferation, migration and inhibits apoptosis in gastric cancer via Akt/mTOR signaling pathway.
Zhang Y, Bai J, Si W, Yuan S, Li Y, Chen X. Zhang Y, et al. Biosci Rep. 2020 Feb 28;40(2):BSR20200041. doi: 10.1042/BSR20200041. Biosci Rep. 2020. PMID: 32109290 Free PMC article.
References
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiological reviews. 1993;73:79–118. - PubMed
- Prasad AS. Impact of the discovery of human zinc deficiency on health. Journal of the American College of Nutrition. 2009;28:257–265. - PubMed
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nature reviews. Neuroscience. 2009;10:780–791. - PubMed
- Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases