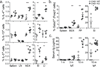Extrathymically generated regulatory T cells control mucosal TH2 inflammation - PubMed (original) (raw)
Extrathymically generated regulatory T cells control mucosal TH2 inflammation
Steven Z Josefowicz et al. Nature. 2012.
Abstract
A balance between pro- and anti-inflammatory mechanisms at mucosal interfaces, which are sites of constitutive exposure to microbes and non-microbial foreign substances, allows for efficient protection against pathogens yet prevents adverse inflammatory responses associated with allergy, asthma and intestinal inflammation. Regulatory T (T(reg)) cells prevent systemic and tissue-specific autoimmunity and inflammatory lesions at mucosal interfaces. These cells are generated in the thymus (tT(reg) cells) and in the periphery (induced (i)T(reg) cells), and their dual origin implies a division of labour between tT(reg) and iT(reg) cells in immune homeostasis. Here we show that a highly selective blockage in differentiation of iT(reg) cells in mice did not lead to unprovoked multi-organ autoimmunity, exacerbation of induced tissue-specific autoimmune pathology, or increased pro-inflammatory responses of T helper 1 (T(H)1) and T(H)17 cells. However, mice deficient in iT(reg) cells spontaneously developed pronounced T(H)2-type pathologies at mucosal sites--in the gastrointestinal tract and lungs--with hallmarks of allergic inflammation and asthma. Furthermore, iT(reg)-cell deficiency altered gut microbial communities. These results suggest that whereas T(reg) cells generated in the thymus appear sufficient for control of systemic and tissue-specific autoimmunity, extrathymic differentiation of T(reg) cells affects commensal microbiota composition and serves a distinct, essential function in restraint of allergic-type inflammation at mucosal interfaces.
Figures
Figure 1. Impaired iTreg cell generation and altered composition of the peripheral Treg cell population in CNS1-deficient mice
a, Relative contribution of CNS1-deficient (GFP+) and -sufficient (GFP−) cells to the Foxp3+ thymocyte subset in 4-day-old CNS1+/− female mice. b, Induction of Foxp3 in Foxp3− TN cells FACS sorted from CNS1− or Foxp3GFP mice stimulated in vitro with TGFβ, IL-2, anti-CD3 and anti-CD28. c, Percent Foxp3+ cells in the spleen, lymph node (LN), mesenteric lymph nodes (MLN), Peyer’s patches (PP) and cells from the small and large intestine lamina propria (SI and LI) of 6–9 month old CNS1− or control mice. d, Percent of transferred CNS1− or CNS1+ CD25−CD44lowCD45.2+OTII+ cells that induced Foxp3 following administration of OVA in water for 6 days. e, Stability of Foxp3 expression in iTreg cells. FACS sorted GFP+ or GFP− cells from Foxp3eGFP-Cre-ERT2 mice were transferred with GFP− or GFP+ cells, respectively, from Ly5.1 Foxp3GFP mice into TCRβδ-deficient recipients. Mice received tamoxifen at 1 (left) or 5 weeks (right) post transfer and stability of Foxp3 expression among YFP-labeled cells was assessed after 4 weeks. All data are representative of two or more independent experiments with n ≥ 3. Error bars represent SD; *, **, and *** indicate p <0.05, 0.01 and 0.001, respectively, as calculated by students’ T-test.
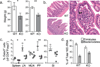
Figure 2. Paucity of iTreg cells results in Th2 inflammation in the gastrointestinal tract
a, Percent of CD4+ cells producing IL-4, IL-13 and IL-5 in 3-month-old mice. b, Percent of Foxp3− CD4+ cells that were Gata3+ in 3-month old mice. c, Concentration of IgE and IgA in serum, determined by enzyme linked immunosorbent assay (ELISA). All data are representative of three or more independent experiments with ≥ 3 mice per group. Error bars represent SD; *, **, and *** indicate p <0.05, 0.01 and 0.001, respectively, as calculated by students’ T-test.
Figure 3. iTreg cells deficiency leads to Th2 type gastrointestinal pathology and altered microbial communities
a, Body weights of 9–12 (left) or 2.5-month-old individually housed (right) CNS1− and littermate control mice (n ≥ 12). b, Plasmacytic enteritis (arrowhead) in CNS1-deficient mice revealed by H&E staining of small intestine from 9–12-month-old CNS1− (bottom and right) and littermate control mice (top). An early crypt abscess is indicated (asterisk). Data represent ≥ 20 mice. c, Percent of Foxp3+ CD4+ cells expressing Gata3+ in 3-month old mice. d, Percent of total 16S rRNA gene sequences of the Firmicutes and Bacteroidetes phyla in stool from individually housed CNS1− (n=9) and WT (n=6) littermate mice. Mean values +/− SEM are shown. All data are representative of three or more independent experiments with ≥ 3 mice per group. Error bars represent SD; *, **, and *** indicate p <0.05, 0.01 and 0.001, respectively, as calculated by students’ T-test. Scale bars = 150 um.
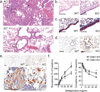
Figure 4. Unprovoked asthma-like airway pathology in CNS1-deficient mice
a, Representative H&E-stained lung section form CNS1− (top) and WT (bottom) mice. The CNS1− lung has marked peribronchiolar inflammation (arrowhead). The reduced lumen (L) contains mucus produced by the hyperplastic respiratory epithelium (E) Arrows indicate reactive (top) and normal (bottom) endothelium. Bottom right hand corner insets are higher magnification of boxed regions and bar indicates smooth muscle thickness. Top right inset (KO) demonstrates eosinophilic crystals. Asterisk marks acidophilic macrophages. b, PAS/AB staining highlighting mucus-producing goblet cells (dark blue-purple) c, Trichrome staining illustrating lung fibrosis (blue staining). d, Arginase-1 staining of lungs from CNS1− and WT mice. “A” indicates airway; an acidophilic crystal is marked by the arrowhead. e, Chitinase 3-like 3 (C3l3) staining of lungs from CNS1− and WT mice at 10× amplification (top) and 60× magnification of lungs from CNS1− mice demonstrating robust C3l3 expression within acidophilic macrophages (bottom). f, Lung resistance (left) and compliance (right) of CNS1− and wild type littermate control mice after exposure to methacholine. Data representative of 2 independent experiments with ≥ 4 mice per group. Error bars represent SD; *, **, and *** indicate p <0.05, 0.01 and 0.001, respectively, as calculated by students’ T-test. Scale bars = 100 um.
Comment in
- A division of labour.
Bordon Y. Bordon Y. Nat Rev Immunol. 2012 Feb 24;12(3):154. doi: 10.1038/nri3180. Nat Rev Immunol. 2012. PMID: 22362347 No abstract available. - Commensal microbiota determine intestinal iTreg.
Alegre ML, Bromberg JS, Bromberg JS. Alegre ML, et al. Am J Transplant. 2012 Aug;12(8):1967. doi: 10.1111/j.1600-6143.2012.04217.x. Am J Transplant. 2012. PMID: 22845904 No abstract available. - From infection to colonization: the role of microbiota in transplantation.
Upadhyay V, Fu YX, Bromberg JS. Upadhyay V, et al. Am J Transplant. 2013 Apr;13(4):829. doi: 10.1111/ajt.12232. Am J Transplant. 2013. PMID: 23551627 Free PMC article. No abstract available.
Similar articles
- Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation.
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Arpaia N, et al. Nature. 2013 Dec 19;504(7480):451-5. doi: 10.1038/nature12726. Epub 2013 Nov 13. Nature. 2013. PMID: 24226773 Free PMC article. - CD4+ Foxp3+ regulatory T cells suppress γδ T-cell effector functions in a model of T-cell-induced mucosal inflammation.
Yurchenko E, Levings MK, Piccirillo CA. Yurchenko E, et al. Eur J Immunol. 2011 Dec;41(12):3455-66. doi: 10.1002/eji.201141814. Epub 2011 Nov 10. Eur J Immunol. 2011. PMID: 21956668 - Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses.
Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Zheng Y, et al. Nature. 2009 Mar 19;458(7236):351-6. doi: 10.1038/nature07674. Epub 2009 Feb 1. Nature. 2009. PMID: 19182775 Free PMC article. - Control of Th2-mediated inflammation by regulatory T cells.
Venuprasad K, Kong YC, Farrar MA. Venuprasad K, et al. Am J Pathol. 2010 Aug;177(2):525-31. doi: 10.2353/ajpath.2010.090936. Epub 2010 Jun 21. Am J Pathol. 2010. PMID: 20566752 Free PMC article. Review. - Antigen-specific CD4(+) regulatory T cells in the intestine.
Tsuji NM. Tsuji NM. Inflamm Allergy Drug Targets. 2006 Sep;5(3):191-201. doi: 10.2174/187152806778256043. Inflamm Allergy Drug Targets. 2006. PMID: 16918482 Review.
Cited by
- Go With Your Gut: The Shaping of T-Cell Response by Gut Microbiota in Allergic Asthma.
Di Gangi A, Di Cicco ME, Comberiati P, Peroni DG. Di Gangi A, et al. Front Immunol. 2020 Jul 14;11:1485. doi: 10.3389/fimmu.2020.01485. eCollection 2020. Front Immunol. 2020. PMID: 32760404 Free PMC article. Review. - Replenishing our defensive microbes.
Ursell LK, Van Treuren W, Metcalf JL, Pirrung M, Gewirtz A, Knight R. Ursell LK, et al. Bioessays. 2013 Sep;35(9):810-7. doi: 10.1002/bies.201300018. Epub 2013 Jul 8. Bioessays. 2013. PMID: 23836415 Free PMC article. Review. - Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases.
Iberg CA, Hawiger D. Iberg CA, et al. Antibodies (Basel). 2020 Jun 15;9(2):23. doi: 10.3390/antib9020023. Antibodies (Basel). 2020. PMID: 32549343 Free PMC article. Review. - Food allergy: an enigmatic epidemic.
Berin MC, Sampson HA. Berin MC, et al. Trends Immunol. 2013 Aug;34(8):390-7. doi: 10.1016/j.it.2013.04.003. Epub 2013 May 4. Trends Immunol. 2013. PMID: 23648309 Free PMC article. Review. - Can we produce true tolerance in patients with food allergy?
Berin MC, Mayer L. Berin MC, et al. J Allergy Clin Immunol. 2013 Jan;131(1):14-22. doi: 10.1016/j.jaci.2012.10.058. J Allergy Clin Immunol. 2013. PMID: 23265693 Free PMC article. Review.
References
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 474(7351):298–306. - PubMed
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. - PubMed
- Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9(2):194–202. - PubMed
Supplemental References
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1(4):1810–1819. - PubMed
- Burich A, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G764–G778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM07739/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- F31 NS073203/NS/NINDS NIH HHS/United States
- 1F31NS073203-01/NS/NINDS NIH HHS/United States
- R37 AI034206/AI/NIAID NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases