Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting - PubMed (original) (raw)
. 2012 Feb 8;482(7385):400-4.
doi: 10.1038/nature10755.
Matthew D Vesely, Daniel C Koboldt, Charles G Rickert, Ravindra Uppaluri, Vincent J Magrini, Cora D Arthur, J Michael White, Yee-Shiuan Chen, Lauren K Shea, Jasreet Hundal, Michael C Wendl, Ryan Demeter, Todd Wylie, James P Allison, Mark J Smyth, Lloyd J Old, Elaine R Mardis, Robert D Schreiber
Affiliations
- PMID: 22318521
- PMCID: PMC3874809
- DOI: 10.1038/nature10755
Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting
Hirokazu Matsushita et al. Nature. 2012.
Abstract
Cancer immunoediting, the process by which the immune system controls tumour outgrowth and shapes tumour immunogenicity, is comprised of three phases: elimination, equilibrium and escape. Although many immune components that participate in this process are known, its underlying mechanisms remain poorly defined. A central tenet of cancer immunoediting is that T-cell recognition of tumour antigens drives the immunological destruction or sculpting of a developing cancer. However, our current understanding of tumour antigens comes largely from analyses of cancers that develop in immunocompetent hosts and thus may have already been edited. Little is known about the antigens expressed in nascent tumour cells, whether they are sufficient to induce protective antitumour immune responses or whether their expression is modulated by the immune system. Here, using massively parallel sequencing, we characterize expressed mutations in highly immunogenic methylcholanthrene-induced sarcomas derived from immunodeficient Rag2(-/-) mice that phenotypically resemble nascent primary tumour cells. Using class I prediction algorithms, we identify mutant spectrin-β2 as a potential rejection antigen of the d42m1 sarcoma and validate this prediction by conventional antigen expression cloning and detection. We also demonstrate that cancer immunoediting of d42m1 occurs via a T-cell-dependent immunoselection process that promotes outgrowth of pre-existing tumour cell clones lacking highly antigenic mutant spectrin-β2 and other potential strong antigens. These results demonstrate that the strong immunogenicity of an unedited tumour can be ascribed to expression of highly antigenic mutant proteins and show that outgrowth of tumour cells that lack these strong antigens via a T-cell-dependent immunoselection process represents one mechanism of cancer immunoediting.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
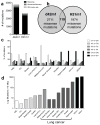
Figure 1. Unedited MCA-induced sarcomas, d42m1 and H31m1, genomically resemble carcinogen-induced human cancers
a, Number of non-synonymous mutations in d42m1 and H31m1 tumour cells as detected by cDNA CapSeq. b, Missense mutations compared between d42m1 and H31m1 that had at least 20x sequencing coverage. c, Spectrum of DNA nucleotide substitutions detected in d42m1 and H31m1 as compared to previously generated data from human cancers including acute myelogenous leukemia (AML), chronic lymphocytic leukemia (CLL), breast cancer (breast-lobular, breast-basal), ovarian cancer (Mardis et al. manuscript in preparation), liver cancer (Hepatitis C Virus (HCV)-positive), melanoma (ultraviolet (UV)-induced), and lung cancers (non-small cell (NSC), small cell (SC), Never-Smoker, Smoker, and Hypermutator (Mardis et al. manuscript in progress). d, Mutation rate for d42m1 and H31m1 and human cancers described in c.
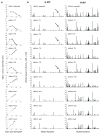
Figure 2. Affinity value profiles of predicted MHC class I epitopes from tumour-specific mutations
a, Growth of d42m1 parental cells, a representative sample of tumour clones, and three escape tumours following transplantation into WT mice (n=5, squares). Data are presented as average tumour diameter ± s.e.m. and are representative of three independent experiments. b, Missense mutations for each d42m1-related tumour examined in (a) were analyzed for potential MHC class I neoepitopes that bind either H-2Db or H-2Kb. Predicted epitope binding affinities were ultimately expressed as “Affinity Values” (Affinity Value = 1/IC50 X 100). Arrow is pointing to a H-2Db epitope created by the R913L spectrin-β2 mutant.
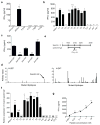
Figure 3. Identification of mutant spectrin-β2 as an authentic antigen of an unedited tumour
a, b, IFN-γ release by C3 CTLs against different unedited sarcomas (a) or against d42m1-related tumours (b). c, IFN-γ release by C3 CTLs is inhibited by mAbs that block CD8 and H-2Db, but not CD4 or H-2Kb. d, MHC class I epitopes predicted to be shared in all of the regressor d42m1 tumours, but not in progressor d42m1 tumours. e, Representation of the cDNA clone that stimulated C3 CTLs encoding the spectrin-β2 R913L mutation. f, qRT-PCR for mutant spectrin-β2 in d42m1-related tumours and 1773. g, IFN-γ release by C3 CTLs incubated with COS-Db cells pulsed with WT (circles) or mutant (squares) spectrin-β2 peptides. Data are representative of three independent experiments. Samples were compared using an unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01, and ***p<0.001; n.s. is non-significant).
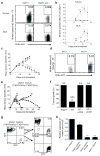
Figure 4. Mutant spectrin-β2 is a major rejection antigen of d42m1
a, Mutant spectrin-β2 specific CD8+ T cells were detected by tetramer staining in tumours and DLNs from mice challenged with d42m1 parental cells, but not d42m1-es3 cells on day 11 post transplant. b, Quantification and kinetics of mutant spectrin-β2 tetramer staining in mice challenged with d42m1 parental cells (n=3, circles) or d42m1-es3 cells (n=3, squares). c, Growth of d42m1-es3 tumour cell clones transduced with WT (n=5, squares) or mutant spectrin-β2 (n=5, circles) and control d42m1-es3 cells (n=5, triangles) following transplantation (1 x 106 cells) into WT mice. Data are presented as average tumour diameter ± s.e.m. d, d42m1-es3 tumours reconstituted with WT (WT.3) or mutant spectrin-β2 (mu.14) were harvested at day 11 and CD8α+ T cells were stained with mutant spectrin-β2 tetramers. e, Growth of a mixture of d42m1-T2RFP (95%) and of d42m1-T3GFP (5%) following transplantation (1 x 106 total cells) into WT (n=5, solid lines, closed squares) or _Rag2_−/− (n=2, dashed lines, open squares) mice. f, Tumour outgrowth in Rag2−/− or WT mice treated or untreated with mAbs that deplete CD4+ or CD8+ T cells following challenge with 1 x 106 cells of a d42m1 mixture (95% d42m1-T2RFP and 5% d42m1-T3GFP). Data presented as percent tumour positive from 2–4 independent experiments (n=2–5 mice per group). g, h, GFP and RFP expression (g) and mutant spectrin-β2 expression (h) were analyzed in the d42m1-T2RFP/d42m1-T3GFP tumour cell mixture before injection and from tumours that grew out in _Rag2_−/− mice (RagPass) or escaped in WT mice by flow cytometry (g) or qRT-PCR (h). Data are representative of two independent experiments. Samples were compared using an unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01, and ***p<0.001; n.s. is non-significant).
Comment in
- Tumour immunogenicity: editorial selection demystified.
Burgess DJ. Burgess DJ. Nat Rev Cancer. 2012 Mar 22;12(4):227. doi: 10.1038/nrc3251. Nat Rev Cancer. 2012. PMID: 22437866 No abstract available. - Tumour immunology: Editorial selection demystified.
Burgess DJ. Burgess DJ. Nat Rev Immunol. 2012 Mar 22;12(4):233. doi: 10.1038/nri3202. Nat Rev Immunol. 2012. PMID: 22437936 No abstract available. - T-cell dependent immunoselection.
Andersen MH. Andersen MH. Oncoimmunology. 2012 Oct 1;1(7):1003. doi: 10.4161/onci.20927. Oncoimmunology. 2012. PMID: 23170248 Free PMC article. No abstract available.
Similar articles
- Expression of tumour-specific antigens underlies cancer immunoediting.
DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. DuPage M, et al. Nature. 2012 Feb 8;482(7385):405-9. doi: 10.1038/nature10803. Nature. 2012. PMID: 22318517 Free PMC article. - Adaptive immunity maintains occult cancer in an equilibrium state.
Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Koebel CM, et al. Nature. 2007 Dec 6;450(7171):903-7. doi: 10.1038/nature06309. Epub 2007 Nov 18. Nature. 2007. PMID: 18026089 - Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy.
Vesely MD, Schreiber RD. Vesely MD, et al. Ann N Y Acad Sci. 2013 May;1284(1):1-5. doi: 10.1111/nyas.12105. Ann N Y Acad Sci. 2013. PMID: 23651186 Free PMC article. - MHC class I antigens, immune surveillance, and tumor immune escape.
Garcia-Lora A, Algarra I, Garrido F. Garcia-Lora A, et al. J Cell Physiol. 2003 Jun;195(3):346-55. doi: 10.1002/jcp.10290. J Cell Physiol. 2003. PMID: 12704644 Review. - Cancer immunoediting: from immunosurveillance to tumor escape.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Dunn GP, et al. Nat Immunol. 2002 Nov;3(11):991-8. doi: 10.1038/ni1102-991. Nat Immunol. 2002. PMID: 12407406 Review.
Cited by
- Longitudinal Immune Profiling Reveals Unique Myeloid and T-cell Phenotypes Associated with Spontaneous Immunoediting in a Prostate Tumor Model.
Ager CR, Obradovic AZ, Arriaga JM, Chaimowitz MG, Califano A, Abate-Shen C, Drake CG. Ager CR, et al. Cancer Immunol Res. 2021 May;9(5):529-541. doi: 10.1158/2326-6066.CIR-20-0637. Epub 2021 Feb 26. Cancer Immunol Res. 2021. PMID: 33637604 Free PMC article. - Combining radiotherapy and cancer immunotherapy: a paradigm shift.
Formenti SC, Demaria S. Formenti SC, et al. J Natl Cancer Inst. 2013 Feb 20;105(4):256-65. doi: 10.1093/jnci/djs629. Epub 2013 Jan 4. J Natl Cancer Inst. 2013. PMID: 23291374 Free PMC article. Review. - A comprehensively prognostic and immunological analysis of actin-related protein 2/3 complex subunit 5 in pan-cancer and identification in hepatocellular carcinoma.
Huang S, Sun L, Hou P, Liu K, Wu J. Huang S, et al. Front Immunol. 2022 Sep 6;13:944898. doi: 10.3389/fimmu.2022.944898. eCollection 2022. Front Immunol. 2022. PMID: 36148220 Free PMC article. - The Prognostic Value of Sex-Determining Region Y-Box 2 and CD8+ Tumor-Infiltrating Lymphocytes in Limited-Stage Small-Cell Lung Cancer.
Lee J, Jung YY, Lee JH, Hong M, Hwang HW, Hong SA, Hong SH. Lee J, et al. Oncology. 2021;99(8):528-538. doi: 10.1159/000516444. Epub 2021 Jun 9. Oncology. 2021. PMID: 34107469 Free PMC article. - Chimeric antigen receptor engineered NK cellular immunotherapy overcomes the selection of T-cell escape variant cancer cells.
Lee MY, Robbins Y, Sievers C, Friedman J, Abdul Sater H, Clavijo PE, Judd N, Tsong E, Silvin C, Soon-Shiong P, Padget MR, Schlom J, Hodge J, Hinrichs C, Allen C. Lee MY, et al. J Immunother Cancer. 2021 Mar;9(3):e002128. doi: 10.1136/jitc-2020-002128. J Immunother Cancer. 2021. PMID: 33741731 Free PMC article.
References
- Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. - PubMed
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. - PubMed
- Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. - PubMed
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural Innate and Adaptive Immunity to Cancer. Annu Rev Immunol. 2011;29:235–271. - PubMed
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 08-0577/AICR_/Worldwide Cancer Research/United Kingdom
- R01 CA043059/CA/NCI NIH HHS/United States
- U01 CA141541/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources