Ebp2 and Brx1 function cooperatively in 60S ribosomal subunit assembly in Saccharomyces cerevisiae - PubMed (original) (raw)
Ebp2 and Brx1 function cooperatively in 60S ribosomal subunit assembly in Saccharomyces cerevisiae
Kaori Shimoji et al. Nucleic Acids Res. 2012 May.
Abstract
The yeast protein Ebp2 is required for early steps in production of 60S ribosomal subunits. To search for cofactors with which Ebp2 functions, or substrates on which it acts, we screened for mutants that were synthetically lethal (sl) with the ebp2-14 mutation. Four different mutant alleles of the 60S ribosomal subunit assembly factor Brx1 were found. To investigate defects of the double mutant, we constructed strains conditional for the ebp2-14 brx1- synthetic lethal phenotype. These ebp2-14 brx1 mutants were defective in processing of 27S pre-rRNA and production of 60S subunits, under conditions where each single mutant was not. Ebp2 and Brx1 exhibit a strong two-hybrid interaction, which is eliminated by some combinations of brx1 and ebp2 mutations. In one such mutant, Ebp2 and Brx1 can still associate with pre-ribosomes, but subunit maturation is perturbed. Depletion of either Ebp2 or Brx1 revealed that Brx1 requires Ebp2 for its stable association with pre-ribosomes, but Ebp2 does not depend on the presence of Brx1 to enter pre-ribosomes. These results suggest that assembly of 60S ribosomal subunits requires cooperation of Ebp2 with Brx1, together with other molecules present in pre-ribosomes, potentially including several found in assembly subcomplexes with Brx1 and Ebp2.
Figures
Figure 1.
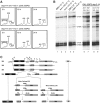
Isolation of mutants exhibiting synthetic lethality with ebp2-14. (A) Schematic representation of the strain used for the sl screen. (B) Non-sectoring phenotype of a mutant. The parent strain (a) and isolated mutants (b) were streaked on YEPD media and incubated at 30°C for 7 days. (C) Cloning of alleles responsible for synthetic lethality with ebp2-14. pKTa3-1 and pKTa3-2 were cloned from a yeast genomic library (cloned in plasmid vector YCplac111), and subcloned into pKTa3-3 (in pRS315) and pKTa3-4 (in YCplac111). The m3 (KM635) strain was transformed with these plasmids or a vector plasmid (pRS315), and cultured on 5-FOA-containing medium at 25°C for 3 days. (D) Synthetic lethal brx1 mutations are in conserved amino acid residues. The amino acid sequences are from the GenBank database. Restricted regions with a homologous sequence are shown in single-letter code; 1, S. cerevisiae (accession Q08235, 30–249 amino acids aligned from full length of 291 amino acids); 2, Schizosaccharomyces pombe (Q9HGL6, 42–255 amino acids from full length of 295 amino acids); 3, Caenorhabditis elegans (P34524, 62–270 amino acids from 352 amino acids); 4, Drosophila melanogaster (Q9VZE6, 49–258 amino acids from full length of 359 amino acids); 5, Xenopus laevis (Q8UVY2, 52–258 amino acids from 339 amino acids); 6, Homo sapiens (Q8TDN6, 59–266 amino acids from 353 amino acids). A sequence alignment was generated by BLAST and ClustalW. Deletions needed for alignment are indicated by dashes. Residues boxed in black are identical in at least three of the sequences compared. The amino acids substituted in the brx1 alleles are indicated with asterisks above the sequence.
Figure 2.
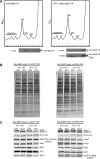
The synthetic lethality of ebp2-14 with brx1. (A) brx1 and ebp2-14 single mutants grow under conditions that are lethal in the double mutants. The wild-type BRX1 strain and brx1-52, brx1-102, brx1-172, brx1-227 and ebp2-14 single mutants were cultured in SC medium at 25°C overnight. Five-fold serial dilutions of the culture (OD600 = ∼1) were spotted onto YEPD medium, and incubated at 16°C for 10 days, and at 25°C, 30°C, 32°C, 35°C and 37°C for 3 days. (B) The temperature-sensitive brx1-227 mutation leu227pro leads to a defect in 60S subunit biogenesis at 37°C. The KM1720 (BRX1) and KM1724 (brx1-227) strains were grown at 25°C overnight. The cultures were then shifted to 37°C and grown for 6 h. Cell extracts were used for polysome analysis by sucrose density gradient ultracentrifugation. Ribosomal profiles were determined by OD254 measurement of the gradient (7–47%) fractions. Arrows indicate halfmer polysomes. (C) Specificity of the synthetic lethality of ebp2-14 with brx1. Tetrad analysis of ebp2 brx1 and rrs1 brx1 diploids_._ Diploids constructed by crossing the ebp2-14 or rrs1-124 mutant with each brx1 mutant were sporulated and meiotic progeny were analyzed by tetrad dissection. Colony formation was scored on plates containing glucose-medium incubated at 25°C for 7–8 days for ebp2-14 (top) or 6 days for rrs1-124 (bottom). Spores corresponding to double mutants are indicated by red triangles.
Figure 3.
Wildtype Ebp2 and Brx1 exhibit a strong two-hybrid interaction, that is disrupted in some of the ebp2 brx1 double mutants. (A) Protein–protein interactions were analyzed by the yeast two-hybrid system. Five-fold serial dilutions of cultures of L40 yeast transformed with lexA binding domain-fused and Gal4p activation domain-fused genes were plated on SC–Trp, Leu, or SC–Trp, Leu, His containing the indicated concentrations of 3-AT, and cultured at 25°C for 4–5 days. (B) Interactions were assayed as described in (A) above.
Figure 4.
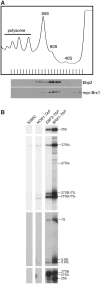
The synergistic defect of ebp2 and brx1 in biogenesis of 60S ribosomal subunits. The single mutant strains KM414 (ebp2-14), KM1722 (brx1-102) and KM1723 (brx1-172), were grown at 25°C and shifted to 37°C for 4 h. The KM1732 (ebp2-14 brx1-102 p_GAL1-HA-EBP2_) and KM1733 (ebp2-14 brx1-172 p_GAL1-HA-EBP_2) strains were grown in galactose medium at 25°C and shifted to glucose medium and grown at 25°C for 16 h and 24 h. (A) Cell extracts were used for polysome analysis by sucrose density gradient ultracentrifugation. (B) Pre-rRNA processing was assayed by primer extension. (C) Pre-rRNA processing pathway.
Figure 5.
Both Brx1 and Ebp2 assemble early into pre-ribosomes. (A) KM1725 (myc-BRX1) cells were grown at 25°C to mid-log phase, then cell extracts were prepared and subjected to sucrose density gradient ultracentrifugation to resolve ribosomal subunits, monoribosomes and polyribosomes. A ribosomal profile was determined by measurement of OD254 of gradient (7–47%) fractions. Each fraction was collected and subjected to SDS–PAGE and western analysis using antibodies against Ebp2 and the myc epitope. (B) Both Ebp2 and Brx1 associate with 90S pre-ribosomes containing 35S pre-rRNA, as well as 66S pre-ribosomes containing 27S pre-rRNAs. TAP-tagged Ebp2 and Brx1 were used to affinity-purify pre-ribosomes. Pre-RNAs contained in these pre-rRNPs were identified by primer extension and gel electrophoresis. The untagged parent strain S288c and Nob1-Tap were used as controls.
Figure 6.
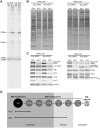
The synthetic lethal mutant ebp2-14 and brx1-102 proteins can assemble into pre-ribosomes, even though they have lost ability to interact with each other in the two-hybrid system. (A) A defect of the mutant ebp2-14 protein at 25°C is revealed by a competition assay. The ebp2–14 mutant protein can assemble into pre-ribosomes, but not when wild-type Ebp2 protein is present. KM1514 (myc-ebp2; left) and wild-type (EBP2) transformed with a plasmid expressing myc-ebp2-14 under the EBP2 promoter (right) were cultured at 25°C. Extracts were prepared from the unshifted and shifted strains and subjected to centrifugation on 7–47% sucrose gradients. Proteins were precipitated from each fraction with 10% TCA. Amounts of wildtype Ebp2 or mutant ebp2 in pre-ribosomes were assayed by western blot analysis using anti-Ebp2 antisera. Anti-myc antibody was used to detect only myc-tagged ebp2-14 protein. (B) The GAL-EBP2 ebp2-14 brx1-102 and GAL-EBP2 ebp2-14 brx1-172 strains containing NOP7-TAP (left), and the GAL-EBP2 ebp2-14 brx1-102 and GAL-EBP2 ebp2-14 brx1-172 strains containing RPF2-TAP (right) were grown in galactose medium at 25°C and shifted to glucose-containing medium for 16 h. Pre-ribosomes were affinity-purified using TAP-tagged assembly factor Nop7 (left) or Rpf2 (right). Proteins present in purified pre-ribosomes were resolved by SDS–PAGE and stained with silver. (C) Amounts of HA-tagged wild-type Ebp2 protein, ebp2-14 mutant protein and myc-tagged brx1 mutant protein were assayed by western blotting using antibodies against Ebp2 and myc. Antibodies against other assembly factors and rp were used as controls. Loading controls are marked with an asterisk. Wild-type HA-tagged Ebp2 could be distinguished from mutant ebp2-14 due to the altered electrophoretic moblity caused by the HA tag.
Figure 7.
Brx1 depends on Ebp2 to assemble into pre-ribosomes, but Ebp2 does not require Brx1. GAL-EBP2 and GAL-BRX1 yeast cells were grown in galactose-containing medium, or shifted to glucose-containing medium for 16 h. (A) The pre-rRNA processing defect in GAL-EBP2 and GAL-BRX1. RNA was extracted from unshifted and shifted strains and assayed by primer extension. (B) SDS–PAGE of proteins in pre-ribosomes purified from strains containing or lacking Ebp2 and Brx1 are shown. Pre-ribosomes were affinity-purified from each culture, using TAP-tagged assembly factor Rpf2 (left) or Rrp5 (right), and proteins in them were resolved by SDS-PAGE and stained with silver. (C) Western blot analysis of association of HA-tagged Ebp2 and HA-tagged Brx1 with pre-ribosomes, upon depletion of Brx1 or Ebp2 respectively. Loading controls are marked with an asterisk. (D) Schematic representation of pre-ribosomal particles co-purifying with Rpf2-Tap and with Rrp5-Tap.
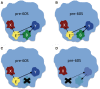
Figure 8.
Model of dependence of Brx1 on Ebp2 for assembly into pre-ribosomes. Ebp2 (E) and Brx1 (B) are represented with yellow and green, respectively. X (red) and Y (blue) represent other proteins present in pre-ribosomes. Each X and each Y can represent one or more proteins. Black lines mark interactions. Loss of interaction is shown as dashed line. Arrows point FROM the protein required to recruit another protein into pre-ribosomes TO the protein dependent on the first protein to be in pre-ribosomes. (A) In the wild-type, Ebp2 and Brx1 interact with each other and each interacts with other proteins within pre-ribosomes (pre-60S). (B) Loss of interaction between Ebp2 and Brx1 does not cause either protein to be absent from pre-ribosomal particles, presumably due their interactions with other proteins. (C) In the absence of Brx1, Ebp2 is still present in pre-ribosomes. (D) The absence of Ebp2 affects one or more proteins that stabilize the association of Brx1 with pre-ribosomes. In their absence, Brx1 is absent from pre-ribosomes.
Similar articles
- Nα-acetyltransferase NatA is involved in ribosome synthesis in Saccharomyces cerevisiae.
Wan K, Tsuchihashi K, Kanda K, Shimoji K, Mizuta K. Wan K, et al. Biosci Biotechnol Biochem. 2013;77(3):631-8. doi: 10.1271/bbb.120860. Epub 2013 Mar 7. Biosci Biotechnol Biochem. 2013. PMID: 23470771 - Roles of Ebp2 and ribosomal protein L36 in ribosome biogenesis in Saccharomyces cerevisiae.
Wan K, Yabuki Y, Mizuta K. Wan K, et al. Curr Genet. 2015 Feb;61(1):31-41. doi: 10.1007/s00294-014-0442-1. Epub 2014 Aug 14. Curr Genet. 2015. PMID: 25119672 - Mak5 and Ebp2 act together on early pre-60S particles and their reduced functionality bypasses the requirement for the essential pre-60S factor Nsa1.
Pratte D, Singh U, Murat G, Kressler D. Pratte D, et al. PLoS One. 2013 Dec 2;8(12):e82741. doi: 10.1371/journal.pone.0082741. eCollection 2013. PLoS One. 2013. PMID: 24312670 Free PMC article. - Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast.
Konikkat S, Woolford JL Jr. Konikkat S, et al. Biochem J. 2017 Jan 15;474(2):195-214. doi: 10.1042/BCJ20160516. Biochem J. 2017. PMID: 28062837 Free PMC article. Review. - The power of AAA-ATPases on the road of pre-60S ribosome maturation--molecular machines that strip pre-ribosomal particles.
Kressler D, Hurt E, Bergler H, Bassler J. Kressler D, et al. Biochim Biophys Acta. 2012 Jan;1823(1):92-100. doi: 10.1016/j.bbamcr.2011.06.017. Epub 2011 Jul 5. Biochim Biophys Acta. 2012. PMID: 21763358 Free PMC article. Review.
Cited by
- Yeast polypeptide exit tunnel ribosomal proteins L17, L35 and L37 are necessary to recruit late-assembling factors required for 27SB pre-rRNA processing.
Gamalinda M, Jakovljevic J, Babiano R, Talkish J, de la Cruz J, Woolford JL Jr. Gamalinda M, et al. Nucleic Acids Res. 2013 Feb 1;41(3):1965-83. doi: 10.1093/nar/gks1272. Epub 2012 Dec 24. Nucleic Acids Res. 2013. PMID: 23268442 Free PMC article. - A protein interaction map of the LSU processome.
McCann KL, Charette JM, Vincent NG, Baserga SJ. McCann KL, et al. Genes Dev. 2015 Apr 15;29(8):862-75. doi: 10.1101/gad.256370.114. Epub 2015 Apr 15. Genes Dev. 2015. PMID: 25877921 Free PMC article. - Ribosomal protein L14 contributes to the early assembly of 60S ribosomal subunits in Saccharomyces cerevisiae.
Espinar-Marchena F, Rodríguez-Galán O, Fernández-Fernández J, Linnemann J, de la Cruz J. Espinar-Marchena F, et al. Nucleic Acids Res. 2018 May 18;46(9):4715-4732. doi: 10.1093/nar/gky123. Nucleic Acids Res. 2018. PMID: 29788267 Free PMC article. - The assembly factor Erb1 functions in multiple remodeling events during 60S ribosomal subunit assembly in S. cerevisiae.
Konikkat S, Biedka S, Woolford JL Jr. Konikkat S, et al. Nucleic Acids Res. 2017 May 5;45(8):4853-4865. doi: 10.1093/nar/gkw1361. Nucleic Acids Res. 2017. PMID: 28115637 Free PMC article. - Stepwise assembly of the earliest precursors of large ribosomal subunits in yeast.
Chen W, Xie Z, Yang F, Ye K. Chen W, et al. Nucleic Acids Res. 2017 Jun 20;45(11):6837-6847. doi: 10.1093/nar/gkx254. Nucleic Acids Res. 2017. PMID: 28402444 Free PMC article.
References
- Raue HA. Pre-ribosomal RNA processing and assembly in Saccharomyces cerevisiae: the machine that makes the machine. In: Olson MOJ, editor. The Nucleolus. NY: Kulwer Academic/Plenum Publishers; 2003. pp. 1–24.
- Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim. Biophys. Acta. 2010;1803:673–683. - PubMed
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M. Crystal structure of the eukaryotic ribosome. Science. 2010;330:1203–1209. - PubMed
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science. 2011;334:941–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases