FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis - PubMed (original) (raw)
FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis
Erin E Mulkearns et al. Mol Biol Cell. 2012 Apr.
Abstract
Clathrin-mediated endocytosis regulates the internalization of many nutrient and signaling receptors. Clathrin and endocytic accessory proteins are recruited to receptors by specific adaptors. The adaptor Disabled-2 (Dab2) recruits its cargoes, including the low-density lipoprotein receptor (LDLR), and mediates endocytosis, even when the major adaptor protein AP2 is depleted. We hypothesized that the accessory proteins normally recruited by AP2 may be recruited by Dab2 if AP2 is absent. We identified one such accessory protein, the F-BAR protein FCH domain only-2 (FCHO2), as a major Dab2-interacting protein. The μ-homology domain (μHD) of FCHO2 binds directly to DPF sequences in Dab2 that also bind AP2. Disrupting the Dab2-FCHO2 interaction inhibited Dab2-mediated LDLR endocytosis in AP2-depleted cells. Depleting FCHO2 reduced the number but increased the size of clathrin structures on the adherent surface of HeLa cells and inhibited LDLR and transferrin receptor clustering. However, LDLR was internalized efficiently by FCHO2-deficient cells when additional time was provided for LDLR to enter the enlarged structures before budding, suggesting that later steps of endocytosis are normal under these conditions. These results indicate FCHO2 regulates the size of clathrin structures, and its interaction with Dab2 is needed for LDLR endocytosis under conditions of low AP2.
Figures
FIGURE 1:
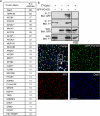
Dab2 interacts with FCHO2. (A) Proteins detected by MS from HBT-Dab2 purification. The total number of peptides is additive from two experiments. *, previously described interaction with Dab2. (B) Coimmunoprecipitation of GFP-FCHO2 with T7-Dab2 transiently expressed in HeLa cells. HeLa cells were lysed 48 h after transfection of GFP-FCHO2 and T7-Dab2 and subjected to immunoprecipitation with anti-T7. (C) HeLa cells transiently transfected with GFP-FCHO2 were fixed, permeabilized, and stained with antibodies to Dab2 and α-adaptin. White areas in the merge panel are places at which all three proteins colocalize. A 0.2-μm-thick section of the adherent surface of the cell is shown. Scale bar: 5 μm.
FIGURE 2:
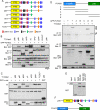
The Dab2 DPF motifs interact with the FCHO2 μHD. (A) Schematic of Dab2 structure and mutants. p96: full-length; p67: splice form lacking amino acids 229–447; NPF*: all five NPFs changed to NPV; ΔC: truncation after amino acid 576; AP2*: two DPFs changed to DAF and FLDLF changed to ALALF; DPF*: two DPFs changed to DAF; FLDLF*: FLDLF changed to ALALF. (B) Coimmunoprecipitation of GFP-FCHO2 with T7-tagged forms of Dab2. HeLa cells were transiently transfected with full-length GFP-FCHO2 and T7-tagged Dab2. Cells were lysed 48 h after transfection and immunoprecipitated with antibody to T7. (C) Binding of Dab2 mutants to AP2-α-ear. Cells were transiently transfected with T7-tagged Dab2 and lysed. Lysates were then mixed with purified, glutathione Sepharose–bound GST-AP2-α-ear. (D) Top, schematic of FCHO2 structure. FCHO2 contains an N-terminal F-BAR domain (residues 1–280), a central area (residues 281–520), and a C-terminal μHD (residues 521–810). FCHO2 constructs and amino acids as follows: A, amino acids 1–280; B, amino acids 1–520; C, amino acids 281–520; D, amino acids 281–810; and E, amino acids 521–810. Bottom, coimmunoprecipitation of GFP-FCHO2 forms with T7-Dab2. HeLa cells were transiently transfected with GFP-FCHO2 and full-length T7-p96. Cells were lysed 48 h after transfection and immunoprecipitated with antibody to T7. Arrowheads indicate GFP-tagged FCHO2 forms that coimmunoprecipitated with T7-Dab2. *, nonspecific band. (E) Binding of purified Dab2 and FCHO2 μHD. Purified, bacterially grown T7-FCHO2 μHD-His was mixed with purified, glutathione Sepharose–bound GST-Dab2 (residues 206–492). (F) The Dab2 DPF motifs (black) interact directly with the FCHO2 μHD.
FIGURE 3:
Disruption of the Dab2-FCHO2 interaction inhibits Dab2-dependent LDLR endocytosis when AP2 is depleted. (A) Dab2 and AP2 are efficiently depleted from HeLa-mini-LDLR cells with siRNA. Cells were transfected with siRNA to Dab2 and AP2 μ2 on days 1 and 3 and lysed for Western blotting on day 5. (B) Dab2-DPF* does not function for LDLR endocytosis in cells with low AP2 levels. HeLa-mini-LDLR cells were transfected with siRNA to Dab2 and AP2 or buffer control. The cells were then transfected with DNA encoding T7-tagged Dab2 p96 or p67 or DPF-mutant p96 (DPF*). Antibody to HA-mini-LDLR was added for 2 min at 37°C to measure receptor uptake. Fixed and permeabilized cells were stained with anti-T7 and appropriate secondary antibodies for anti-T7 (bottom row) and internalized anti-HA (top row). Only cells that were transfected with T7-Dab2 contain specific T7 staining; nuclear staining is background. Images are Z-stack projections. Scale bar: 10 μm. (C) Means and SEs of fluorescence intensity of at least five cells from three separate experiments are shown. *, p < 0.05 by Student's t test compared with control cells. Dashed line indicates control level; EV: empty vector. (D) Dab2-DPF* functions normally for LDLR endocytosis in cells with normal AP2. HeLa-mini-LDLR cells were transfected with siRNA to Dab2 and ARH or buffer control. The cells were then transfected with DNA encoding T7-tagged Dab2 p96 or p67 or DPF-mutant p96 (DPF*). Antibody to HA-mini-LDLR was added for 2 min at 37°C to measure receptor uptake. Fixed and permeabilized cells were stained with anti-T7 and appropriate secondary antibodies for anti-T7 (bottom row) and internalized anti-HA (top row). Images are Z-stack projections. Scale bar: 10 μm. (E) Means and SEs of fluorescence intensity of at least five cells from three separate experiments are shown. *, p < 0.05 by Student's t test compared with control cells. Dashed line indicates control level; EV: empty vector.
FIGURE 4:
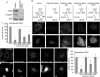
FCHO2 depletion affects the organization of CCP components and inhibits CME. (A) FCHO2 siRNA efficiently depletes FCHO2 from HeLa cells. Left, HeLa-mini-LDLR cells were transiently transfected with siRNA on days 1 and 3 and analyzed for mRNA levels by RT-PCR on day 5. Right, FCHO2 siRNA depletes GFP-FCHO2 from HeLa cells. Cells were transfected with FCHO2 siRNA on day 1, GFP-FCHO2 DNA on day 2, and FCHO2 siRNA again on day 3, and were lysed for Western blot on day 4. (B) Depletion of FCHO2 by siRNA induces large structures in HeLa cells. HeLa cells transiently transfected with FCHO2 siRNA and LCa-GFP were permeabilized and stained with antibodies to Dab2 and AP2. The bottom surface of the cell is shown. Scale bar: 10 μm. (C) Distribution of plaque size in FCHO2-depleted cells shows an increase in the occurrence of large structures on their adherent surfaces compared with control cells. The size of Dab2-positive structures with diameters larger than 220 nm was measured using ImageJ. The data shown is from a representative experiment. p value was calculated using the Mann-Whitney U test. (D and E) Depletion of FCHO2 by siRNA inhibits HA-mini-LDLR (D) and TfnR (E) endocytosis. Cells were given antibody against HA or TfnR and allowed to internalize for 2 min at 37°C. Values are mean fluorescence intensity ± SE of three experiments. Images are Z-stack projections. Scale bars: 20 μm. *, p < 0.05 by Student's t test compared with control. (F and G) Surface HA-mini-LDLR (F) and TfnR (G) fail to cluster into CCSs in FCHO2-depleted cells. Nonpermeabilized cells were stained with antibody to the extracellular domain of receptors, permeabilized, and stained with antibody to Dab2. Images are of the adherent surface of cells; blue is DAPI staining. Scale bars: 10 μm. (H) Colocalization of LDLR and TfnR with Dab2 at steady state. Using ImageJ, the percent area of surface receptor that colocalized with Dab2 was measured and mean and SEs were plotted. *, p < 0.05 by Student's t test.
FIGURE 5:
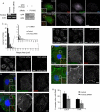
Endocytic activity of enlarged CCSs. (A) Incubation at 4°C allows HA-mini-LDLR to cluster into enlarged plaques. Control or FCHO2 siRNA-treated HeLa-mini-LDLR cells were fixed, stained with antibody to HA, permeabilized, and then stained with antibody to Dab2. Images are adherent surface of cells. Scale bar: 10 μm. (aCHO2 is dispensable for LDLR endocytosis when cells are preincubated at 4°C for 1 h. HeLa mini-LDLR cells were incubated with antibody to HA for 1 h at 4°C, washed, warmed to 37°C for 2 min, then cooled, acid-stripped, and permeabilized, and HA-LDLR uptake was visualized using immunofluorescence. Values are mean fluorescence intensity ± SE of three experiments. Images are Z-stack projections. Scale bar: 20 μm. *, p < 0.05 by Student's t test. (C) TfnR fails to localize to enlarged CCSs with a 4°C incubation. Control or FCHO2 siRNA-treated HeLa-mini-LDLR cells were fixed, stained with antibody to TfnR, permeabilized, and then stained with antibody to Dab2. Images are adherent surface of cells. Scale bar: 10 μm. (D) Depletion of FCHO2 inhibits TfnR endocytosis even with a 4°C preincubation. HeLa-mini-LDLR cells were incubated with antibody to TfnR for 1 h at 4°C, washed, and moved to 37°C for 2 min to measure TfnR uptake. Values are mean fluorescence intensity ± SE of three experiments. Images are Z-stack projections. Scale bar: 20 μm. *, p < 0.05 by Student's t test. (E) Colocalization of LDLR and TfnR with Dab2 after 1 h at 4°C incubation. Using ImageJ, the percent of surface receptor area that colocalized with Dab2 area was measured, and mean and SEs were plotted. *, p < 0.05 by Student's t test. (F) Receptors are internalized from enlarged structures. HeLa-mini-LDLR cells were given antibody to HA-mini-LDLR for 1 h at 4°C and washed, and then were either fixed (1 h at 4°C) or warmed to 37°C for 2 min and fixed. Nonpermeabilized cells were stained with secondary antibody to surface HA-labeled receptor and then permeabilized and stained with anti-Dab2 and secondary antibodies for anti-Dab2 and internalized anti-HA–labeled receptor. Numbers on HA surface panels are mean fluorescence intensity ± SE of surface HA that colocalized with Dab2. Scale bar: 10 μm. *, p < 0.05 by Student's t test, compared with control after 1 h at 4°C.
Similar articles
- The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin β1 endocytosis.
Teckchandani A, Mulkearns EE, Randolph TW, Toida N, Cooper JA. Teckchandani A, et al. Mol Biol Cell. 2012 Aug;23(15):2905-16. doi: 10.1091/mbc.E11-12-1007. Epub 2012 May 30. Mol Biol Cell. 2012. PMID: 22648170 Free PMC article. - The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH.
Maurer ME, Cooper JA. Maurer ME, et al. J Cell Sci. 2006 Oct 15;119(Pt 20):4235-46. doi: 10.1242/jcs.03217. Epub 2006 Sep 19. J Cell Sci. 2006. PMID: 16984970 - The clathrin adaptor proteins ARH, Dab2, and numb play distinct roles in Niemann-Pick C1-Like 1 versus low density lipoprotein receptor-mediated cholesterol uptake.
Wei J, Fu ZY, Li PS, Miao HH, Li BL, Ma YT, Song BL. Wei J, et al. J Biol Chem. 2014 Nov 28;289(48):33689-700. doi: 10.1074/jbc.M114.593764. Epub 2014 Oct 20. J Biol Chem. 2014. PMID: 25331956 Free PMC article. - Cargo recognition during clathrin-mediated endocytosis: a team effort.
Sorkin A. Sorkin A. Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review. - Regulation of Clathrin-Mediated Endocytosis.
Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Mettlen M, et al. Annu Rev Biochem. 2018 Jun 20;87:871-896. doi: 10.1146/annurev-biochem-062917-012644. Epub 2018 Apr 16. Annu Rev Biochem. 2018. PMID: 29661000 Free PMC article. Review.
Cited by
- Dab2 gene variant is associated with increased coronary artery disease risk in Chinese Han population.
Wang Y, Wang Y, Adi D, He X, Liu F, Abudesimu A, Fu Z, Ma Y. Wang Y, et al. Medicine (Baltimore). 2020 Jul 2;99(27):e20924. doi: 10.1097/MD.0000000000020924. Medicine (Baltimore). 2020. PMID: 32629690 Free PMC article. - Immune microenvironment-related gene mapping predicts immunochemotherapy response and prognosis in diffuse large B-cell lymphoma.
Chen W, Liang W, He Y, Liu C, Chen H, Lv P, Yao Y, Zhou H. Chen W, et al. Med Oncol. 2022 Jan 29;39(4):44. doi: 10.1007/s12032-021-01642-3. Med Oncol. 2022. PMID: 35092504 - Caenorhabditis elegans reveals a FxNPxY-independent low-density lipoprotein receptor internalization mechanism mediated by epsin1.
Kang YL, Yochem J, Bell L, Sorensen EB, Chen L, Conner SD. Kang YL, et al. Mol Biol Cell. 2013 Feb;24(3):308-18. doi: 10.1091/mbc.E12-02-0163. Epub 2012 Dec 14. Mol Biol Cell. 2013. PMID: 23242996 Free PMC article. - DNA methylation microarrays identify epigenetically regulated lipid related genes in obese patients with hypercholesterolemia.
Płatek T, Polus A, Góralska J, Raźny U, Gruca A, Kieć-Wilk B, Zabielski P, Kapusta M, Słowińska-Solnica K, Solnica B, Malczewska-Malec M, Dembińska-Kieć A. Płatek T, et al. Mol Med. 2020 Oct 7;26(1):93. doi: 10.1186/s10020-020-00220-z. Mol Med. 2020. PMID: 33028190 Free PMC article. - Endocytosis and Physiology: Insights from Disabled-2 Deficient Mice.
Tao W, Moore R, Smith ER, Xu XX. Tao W, et al. Front Cell Dev Biol. 2016 Nov 25;4:129. doi: 10.3389/fcell.2016.00129. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27933291 Free PMC article. Review.
References
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials