Myosin-V as a mechanical sensor: an elastic network study - PubMed (original) (raw)
Myosin-V as a mechanical sensor: an elastic network study
Markus Düttmann et al. Biophys J. 2012.
Abstract
According to recent experiments, the molecular-motor myosin behaves like a strain sensor, exhibiting different functional responses when loads in opposite directions are applied to its tail. Within an elastic-network model, we explore the sensitivity of the protein to the forces acting on the tail and find, in agreement with experiments, that such forces invoke conformational changes that should affect filament binding and ADP release. Furthermore, conformational responses of myosin to the application of forces to individual residues in its principal functional regions are systematically investigated and a detailed sensitivity map of myosin-V is thus obtained. The results suggest that the strain-sensor behavior is involved in the intrinsic operation of this molecular motor.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
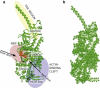
Figure 1
Myosin-V (a) and its EN (b). In panel a, three principal functional regions of the protein are schematically shown; the ATP-analog is also displayed (orange). Moreover, some important structural elements, such as the front and the back doors and the HCM loop, are also indicated here. In panel b, each particle corresponds to a residue, the links represent elastic interactions between them.
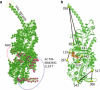
Figure 2
(a) Set of residues probed by application of static forces. (b) Labels, and the distances between them, used to monitor conformational changes.
Figure 3
Responses of pair distances between labels 115 and 297 (black), 219 and 442 (red), 343 and 517 (blue), and 386 and 517 (green) as functions of the amplitude of the force applied to the tail. Small abrupt changes observed at large negative forces are due to local buckling effects.
Figure 4
Sensitivity of residues in the NBP region. Directions of communication are indicated by colored arrows. (Inset) Enlargement of the region of the NBP outlined by the box. Residues most sensitive with respect to tail motion and conformational changes in the actin cleft are colored red. Responses induced by the forces applied at the gray colored residues are weaker.
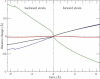
Figure 5
Changes in the distance between residues 343 and 517, which characterize opening of the actin-binding cleft, as functions of the force applied to residue 442 in the back-door region. The red curve corresponds to the forward force of 5 Å applied to the tail. The green curve is for the force with the same strength applied in the backward direction. The blue curve corresponds to the absence of force.
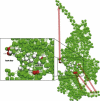
Figure 6
Responses induced by the application of forces to residues in the actin-binding cleft region. Red spheres indicate residues 377–390 in the HCM loop, 340–350 in the upper 50-kDa subdomain, and 540–544 in the lower 50-kDa subdomain. Gray spheres represent less sensitive residues in the lower 50-kDa subdomain. Arrows schematically indicate the directions and strength of intramolecular communication.
Similar articles
- Influence of fluctuations in actin structure on myosin V step size.
Vilfan A. Vilfan A. J Chem Inf Model. 2005 Nov-Dec;45(6):1672-5. doi: 10.1021/ci050182m. J Chem Inf Model. 2005. PMID: 16309271 - Force-producing ADP state of myosin bound to actin.
Wulf SF, Ropars V, Fujita-Becker S, Oster M, Hofhaus G, Trabuco LG, Pylypenko O, Sweeney HL, Houdusse AM, Schröder RR. Wulf SF, et al. Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):E1844-52. doi: 10.1073/pnas.1516598113. Epub 2016 Mar 14. Proc Natl Acad Sci U S A. 2016. PMID: 26976594 Free PMC article. - Holding the reins on myosin V.
Olivares AO, De La Cruz EM. Olivares AO, et al. Proc Natl Acad Sci U S A. 2005 Sep 27;102(39):13719-20. doi: 10.1073/pnas.0507068102. Epub 2005 Sep 19. Proc Natl Acad Sci U S A. 2005. PMID: 16172373 Free PMC article. Review. No abstract available. - Regulation and recycling of myosin V.
Taylor KA. Taylor KA. Curr Opin Cell Biol. 2007 Feb;19(1):67-74. doi: 10.1016/j.ceb.2006.12.014. Epub 2007 Jan 8. Curr Opin Cell Biol. 2007. PMID: 17208425 Review.
Cited by
- Allosteric communication in molecular machines via information exchange: what can be learned from dynamical modeling.
Loutchko D, Flechsig H. Loutchko D, et al. Biophys Rev. 2020 Apr;12(2):443-452. doi: 10.1007/s12551-020-00667-8. Epub 2020 Mar 20. Biophys Rev. 2020. PMID: 32198636 Free PMC article. Review. - Coarse-Grained Protein Dynamics Studies Using Elastic Network Models.
Togashi Y, Flechsig H. Togashi Y, et al. Int J Mol Sci. 2018 Dec 5;19(12):3899. doi: 10.3390/ijms19123899. Int J Mol Sci. 2018. PMID: 30563146 Free PMC article. Review. - Membrane trafficking in podocyte health and disease.
Swiatecka-Urban A. Swiatecka-Urban A. Pediatr Nephrol. 2013 Sep;28(9):1723-37. doi: 10.1007/s00467-012-2281-y. Epub 2012 Aug 30. Pediatr Nephrol. 2013. PMID: 22932996 Free PMC article. Review. - Effective harmonic potentials: insights into the internal cooperativity and sequence-specificity of protein dynamics.
Dehouck Y, Mikhailov AS. Dehouck Y, et al. PLoS Comput Biol. 2013;9(8):e1003209. doi: 10.1371/journal.pcbi.1003209. Epub 2013 Aug 29. PLoS Comput Biol. 2013. PMID: 24009495 Free PMC article. - Molecular mechanisms underlying the impact of mutations in SOD1 on its conformational properties associated with amyotrophic lateral sclerosis as revealed with molecular modelling.
Alemasov NA, Ivanisenko NV, Ramachandran S, Ivanisenko VA. Alemasov NA, et al. BMC Struct Biol. 2018 Feb 5;18(Suppl 1):1. doi: 10.1186/s12900-018-0080-9. BMC Struct Biol. 2018. PMID: 29431095 Free PMC article.
References
- Sellers J.R. 2nd ed. Oxford University Press; New York: 1999. Myosins.
- Sweeney H.L., Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu. Rev. Biophys. 2010;39:539–557. - PubMed
- Iwaki M., Iwane A.H., Yanagida T. Brownian search-and-catch mechanism for myosin-VI steps. Nat. Chem. Biol. 2009;5:403–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources