Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma - PubMed (original) (raw)
Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma
Gulab S Zode et al. Invest Ophthalmol Vis Sci. 2012.
Abstract
Purpose: Mutations in the myocilin gene (MYOC) are the most common known genetic cause of primary open-angle glaucoma (POAG). The purpose of this study was to determine whether topical ocular sodium 4-phenylbutyrate (PBA) treatment rescues glaucoma phenotypes in a mouse model of myocilin-associated glaucoma (Tg-MYOC(Y437H) mice).
Methods: Tg-MYOC(Y437H) mice were treated with PBA eye drops (n = 10) or sterile PBS (n = 8) twice daily for 5 months. Long-term safety and effectiveness of topical PBA (0.2%) on glaucoma phenotypes were examined by measuring intraocular pressure (IOP) and pattern ERG (PERG), performing slit lamp evaluation of the anterior chamber, analyzing histologic sections of the anterior segment, and comparing myocilin levels in the aqueous humor and trabecular meshwork of Tg-MYOC(Y437H) mice.
Results: Tg-MYOC(Y437H) mice developed elevated IOP at 3 months of age when compared with wild-type (WT) littermates (n = 24; P < 0.0001). Topical PBA did not alter IOP in WT mice. However, it significantly reduced elevated IOP in Tg-MYOC(Y437H) mice to the level of WT mice. Topical PBA-treated Tg-MYOC(Y437H) mice also preserved PERG amplitudes compared with vehicle-treated Tg-MYOC(Y437H) mice. No structural abnormalities were observed in the anterior chamber of PBA-treated WT and Tg-MYOC(Y437H) mice. Analysis of the myocilin in the aqueous humor and TM revealed that PBA significantly improved the secretion of myocilin and reduced myocilin accumulation as well as endoplasmic reticulum (ER) stress in the TM of Tg-MYOC(Y437H) mice. Furthermore, topical PBA reduced IOP elevated by induction of ER stress via tunicamycin injections in WT mice.
Conclusions: Topical ocular PBA reduces glaucomatous phenotypes in Tg-MYOC(Y437H) mice, most likely by reducing myocilin accumulation and ER stress in the TM. Topical ocular PBA could become a novel treatment for POAG patients with myocilin mutations.
Figures
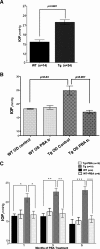
Figure 1.
Topical ocular PBA rescues ocular hypertension in Tg-MYOCY437H mice. (A) IOP measurements of 3-month-old WT and Tg-MYOCY437H mice. Tg-MYOCY437H mice developed elevated IOP compared with WT littermates. (B) Topical ocular PBA reduced IOP in the left eye compared with the contralateral control eye in 9-month-old Tg-MYOCY437H mice. Topical ocular PBA was applied to the left eye of Tg-MYOCY437H mice, and the right eye served as the control. One week after PBA treatment, IOP in the left eye was significantly reduced compared with the contralateral eye. n = 3 WT and n = 5 Tg-MYOCY437H mice. (C) IOP measurements of vehicle or PBA-treated WT and Tg-MYOCY437H mice for 5 months. WT and Tg-MYOCY437H mice (3 months old) were divided into two groups: the first received topical ocular PBA (0.2%) twice daily, and the second was given sterile PBS (vehicle) twice daily. The IOP of these mice was measured every month. Vehicle-treated Tg-MYOCY437H mice showed elevated IOP compared with that of the WT littermates; however, PBA treatment normalized the IOP of the Tg-MYOCY437H mice to WT levels. *P < 0.05, **P < 0.01, ***P < 0.0001, versus vehicle-treated Tg-MYOCY437H mice. Data are the mean ± SEM.
Figure 2.
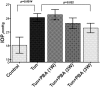
Topical ocular PBA treatment prevents retinal ganglion cells functional deficits in Tg-MYOCY437H mice. PERG amplitudes (P50–N95, μV) were measured to evaluate the functional deficit in the RGCs of Tg-MYOCY437H mice treated with topical PBA. PBA treatment of Tg-MYOCY437H mice for 5 months prevented a reduction in PERG amplitudes compared with those in vehicle-treated Tg-MYOCY437H mice (∼50% loss in PERG amplitudes). n = 6 vehicle-treated WT, n = 5 PBA treated WT, n = 12 vehicle-treated Tg-MYOCY437H, and n = 6 PBA-treated Tg-MYOCY437H mice. Data are the mean ± SEM.
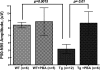
Figure 3.
Topical ocular PBA does not cause abnormalities to anterior segment structures in WT or Tg-MYOCY437H mice. Slit lamp examination of PBA-treated WT (A) and Tg-MYOCY437H (B) mice revealed no abnormalities in the anterior segment structures (iris, pupil, lens, and cornea). H&E staining of PBA-treated WT (C) and Tg-MYOCY437H (D) mice. Optical coherence tomography (OCT) shows no abnormalities in cornea of PBA-treated Tg-MYOCY437H mice (F) compared with WT littermates (E). Cornea thickness measurements are shown in (G). n = 4 WT and Tg-MYOCY437H treated with PBA or vehicle. Data are the mean ± SEM.
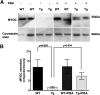
Figure 4.
Topical ocular PBA reverses inhibition of myocilin secretion in the aqueous humor of Tg-MYOCY437H mice. (A) Western blot analysis of myocilin in the aqueous humor samples from 9-month-old PBA-treated WT and Tg-MYOCY437H mice is compared with vehicle-treated WT and Tg-MYOCY437H mice. Coomassie stain was performed to ensure equal loading of aqueous humor. (B) Densitometric analysis of myocilin secretion normalized to a loading control demonstrated a significant reduction in myocilin secretion in the Tg-MYOCY437H mice compared with that in WT littermates; however, PBA treatment of Tg-MYOCY437H mice significantly enhanced myocilin secretion in the aqueous humor. n = 3 WT, n =3 PBA-treated WT, n =5 Tg-MYOCY437H, and n =4 PBA-treated Tg-MYOCY437H mice.
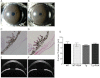
Figure 5.
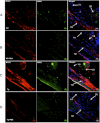
Topical ocular PBA reduces intracellular accumulation of myocilin and ER stress in the TM of Tg-MYOCY437H mice. Myocilin levels and ER stress markers in the iridocorneal angle of PBA-treated WT (B) and Tg-MYOCY437H (D) mice were compared with those in vehicle-treated WT (A) and Tg-MYOCY437H mice (C) by immunostaining and confocal imaging. Arrows: TM, CB, and iris. Vehicle-treated Tg-MYOCY437H mice demonstrated increased myocilin staining and ER stress markers (the KDEL antibody recognizes GRP78 and GRP94) in the TM and CB compared with that in the WT mice. Of note, PBA-treated Tg-MYOCY437H mice showed reduction of myocilin staining in the TM and CB compared with vehicle-treated Tg-MYOCY437H mice. n = 3 vehicle-treated WT, n = 5 vehicle-treated Tg-MYOCY437H, n = 3 PBA-treated WT, and n = 4 PBA-treated Tg-MYOCY437H mice. Scale bar, 20 μm.
Figure 6.
Topical PBA reduces IOP that has been elevated by tunicamycin in WT mice. WT mice (n = 14) injected with 0.2 μg/eye of tunicamycin had elevated IOP 1 week after injection, compared with IOP in WT mice with control vehicle injection (n = 6). Tunicamycin-injected mice that showed elevated IOP were treated with topical PBA eye drops (1%) twice daily afterward, and IOP was measured every week. A 3-week treatment with PBA significantly reduced the elevated IOP in the WT mice. Data are the mean ± SEM.
Similar articles
- Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma.
Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Zode GS, et al. J Clin Invest. 2011 Sep;121(9):3542-53. doi: 10.1172/JCI58183. Epub 2011 Aug 8. J Clin Invest. 2011. PMID: 21821918 Free PMC article. - Expression of Mutant Myocilin Induces Abnormal Intracellular Accumulation of Selected Extracellular Matrix Proteins in the Trabecular Meshwork.
Kasetti RB, Phan TN, Millar JC, Zode GS. Kasetti RB, et al. Invest Ophthalmol Vis Sci. 2016 Nov 1;57(14):6058-6069. doi: 10.1167/iovs.16-19610. Invest Ophthalmol Vis Sci. 2016. PMID: 27820874 Free PMC article. - Intraocular pressure across the lifespan of Tg-MYOCY437H mice.
Zhang X, Xi G, Feng P, Li C, Kuehn MH, Zhu W. Zhang X, et al. Exp Eye Res. 2024 Apr;241:109855. doi: 10.1016/j.exer.2024.109855. Epub 2024 Mar 5. Exp Eye Res. 2024. PMID: 38453040 - Targeting the ER-autophagy system in the trabecular meshwork to treat glaucoma.
Stothert AR, Fontaine SN, Sabbagh JJ, Dickey CA. Stothert AR, et al. Exp Eye Res. 2016 Mar;144:38-45. doi: 10.1016/j.exer.2015.08.017. Epub 2015 Aug 22. Exp Eye Res. 2016. PMID: 26302411 Free PMC article. Review. - A review of genetic and structural understanding of the role of myocilin in primary open angle glaucoma.
Kanagavalli J, Pandaranayaka E, Krishnadas SR, Krishnaswamy S, Sundaresan P. Kanagavalli J, et al. Indian J Ophthalmol. 2004 Dec;52(4):271-80. Indian J Ophthalmol. 2004. PMID: 15693317 Review.
Cited by
- Genes and genetics in eye diseases: a genomic medicine approach for investigating hereditary and inflammatory ocular disorders.
Singh M, Tyagi SC. Singh M, et al. Int J Ophthalmol. 2018 Jan 18;11(1):117-134. doi: 10.18240/ijo.2018.01.20. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29376001 Free PMC article. Review. - Endoplasmic Reticulum Stress Disrupts Mitochondrial Bioenergetics, Dynamics and Causes Corneal Endothelial Cell Apoptosis.
Qureshi S, Lee S, Steidl W, Ritzer L, Parise M, Chaubal A, Kumar V. Qureshi S, et al. Invest Ophthalmol Vis Sci. 2023 Nov 1;64(14):18. doi: 10.1167/iovs.64.14.18. Invest Ophthalmol Vis Sci. 2023. PMID: 37962528 Free PMC article. - Drug Discovery Strategies for Inherited Retinal Degenerations.
Das A, Imanishi Y. Das A, et al. Biology (Basel). 2022 Sep 10;11(9):1338. doi: 10.3390/biology11091338. Biology (Basel). 2022. PMID: 36138817 Free PMC article. Review. - Genome-Wide RNA Sequencing of Human Trabecular Meshwork Cells Treated with TGF-β1: Relevance to Pseudoexfoliation Glaucoma.
Roodnat AW, Callaghan B, Doyle C, Henry M, Goljanek-Whysall K, Simpson DA, Sheridan C, Atkinson SD, Willoughby CE. Roodnat AW, et al. Biomolecules. 2022 Nov 15;12(11):1693. doi: 10.3390/biom12111693. Biomolecules. 2022. PMID: 36421707 Free PMC article. - Inhibition of endoplasmic reticulum stress by 4-phenylbutyrate alleviates retinal inflammation and the apoptosis of retinal ganglion cells after ocular alkali burn in mice.
Huang Y, Yuan M, Duan F, Yang Y, Lou B, Lin X. Huang Y, et al. Inflamm Res. 2022 Jun;71(5-6):577-590. doi: 10.1007/s00011-022-01565-3. Epub 2022 Apr 12. Inflamm Res. 2022. PMID: 35415762
References
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57 - PubMed
- Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670 - PubMed
- Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47:547–561 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY10564/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 EY018825/EY/NEI NIH HHS/United States
- R01 EY010564/EY/NEI NIH HHS/United States
- R01 EY017673/EY/NEI NIH HHS/United States
- F32 EY021436/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous