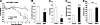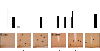Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway - PubMed (original) (raw)
. 2012 May;26(5):2187-96.
doi: 10.1096/fj.11-199067. Epub 2012 Feb 13.
Ryo Suzuki, Tian Lian Huang, Enxuan Jing, Tim J Schulz, Kevin Lee, Cullen M Taniguchi, Daniel O Espinoza, Lindsay E McDougall, Hongbin Zhang, Tong-Chuan He, Efi Kokkotou, Yu-Hua Tseng
Affiliations
- PMID: 22331196
- PMCID: PMC3336788
- DOI: 10.1096/fj.11-199067
Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway
Kristy L Townsend et al. FASEB J. 2012 May.
Abstract
Body weight is regulated by coordinating energy intake and energy expenditure. Transforming growth factor β (TGFβ)/bone morphogenetic protein (BMP) signaling has been shown to regulate energy balance in lower organisms, but whether a similar pathway exists in mammals is unknown. We have previously demonstrated that BMP7 can regulate brown adipogenesis and energy expenditure. In the current study, we have uncovered a novel role for BMP7 in appetite regulation. Systemic treatment of diet-induced obese mice with BMP7 resulted in increased energy expenditure and decreased food intake, leading to a significant reduction in body weight and improvement of metabolic syndrome. Similar degrees of weight loss with reduced appetite were also observed in BMP7-treated ob/ob mice, suggesting a leptin-independent mechanism utilized by BMP7. Intracerebroventricular administration of BMP7 to mice led to an acute decrease in food intake, which was mediated, at least in part, by a central rapamycin-sensitive mTOR-p70S6 kinase pathway. Together, these results underscore the importance of BMP7 in regulating both food intake and energy expenditure, and suggest new therapeutic approaches for obesity and its comorbidities.
Figures
Figure 1.
Systemic administration of BMP7 reduces appetite and body weight in DIO mice. A) Body weight monitoring of DIO mice receiving BMP7 or LacZ adenovirus (control), showing a weight-lowering effect of BMP7. B) DIO mice treated with BMP7 adenovirus had increased oxygen consumption as measured by CLAMS, compared to mice treated with LacZ adenovirus. C) DIO mice treated with BMP7 adenovirus consumed less food during a 12-d period, compared to mice treated with LacZ adenovirus. D) DIO mice treated with BMP7 adenovirus lost more weight compared to pair-fed animals, indicating that the effects of BMP7 on body weight reflect both increased energy expenditure and reduced food intake. E) BMP7-mediated weight loss in DIO mice was due to a decrease in fat mass, not lean mass, as measured by DEXA scanning. F) BMP7-treated DIO mice had lower serum leptin levels at the end of the study, consistent with reduced adiposity in these mice, compared to DIO mice treated with LacZ adenovirus. G, H) DIO mice treated with BMP7 adenovirus had improved glucose tolerance and increased insulin sensitivity as evaluated by glucose (G) and insulin (H) tolerance tests. I) H&E-stained liver sections of DIO mice treated with BMP7 or LacZ for 12 d. Mice treated with BMP7 (right panel) had reduced fat accumulation in their liver compared to control mice (left panel). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
Systemic effects of BMP7 are leptin-independent. A, B) Treatment of leptin-deficient ob/ob mice with BMP7 adenovirus resulted in significant weight loss (A) and reduced food consumption (B) compared to ob/ob mice that received LacZ adenovirus (control) during a 13-d study. C–E) BMP7 adenovirus-treated mice had lower plasma insulin levels (C), higher adiponectin levels (D), and no change in glucose levels (E), indicating increased insulin sensitivity compared to LacZ-adenovirus-treated ob/ob mice. N.S., not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Figure 3.
Expression of BMP7 ligand and receptors in the hypothalamus. A) In situ hybridization analysis of hypothalamic sections for BMPRII (pink), showing caudal hypothalamus (left panel) and rostral hypothalamus (right panel). Antisense represents specific staining (top panel), while sense staining represents background (bottom panel). BMPRII, a type 2 BMP receptor, was highly expressed throughout the hypothalamus. B, C) Immunofluorescence staining (green FITC signal) for the type 1 BMP receptors ALK3 (B) and ALK2 (C) in the mouse hypothalamus, along the third ventricle. ALK3 staining was most robust in the arcuate and paraventricular nuclei, while ALK2 staining was most robust in the dorsomedial and paraventricular nuclei. D) LacZ staining of mouse hypothalamus (top panel) and dorsal midbrain (including ventricular system and choroid plexus; bottom panel) showing BMP7-positive cells (blue) in BMP7 reporter mice. Arrowheads indicate choroid plexus surrounding the ventricles (bottom panel).
Figure 4.
Intracerebroventricular administration of BMP7 suppresses food intake. A) Intracerebroventricular administration of rhBMP7 acutely reduced food intake in C57BL/6 mice. Results are presented from a representative of 7 independent experiments. Leptin served as a positive control. B) Food intake 4 h after i.c.v. rhBMP7 or vehicle treatment of ob/ob mice, showing that leptin-deficient mice maintain an anorectic response to BMP7 administration. C) Hypothalamic staining for c-Fos immunoreactivity (brown) at 60 min after i.c.v. injection of mice with either vehicle (left panel), rhBMP7 (middle panel), or leptin (right panel), showing increased c-Fos expression in response to both leptin and BMP7 treatments in the arcuate and ventromedial nuclei. D) mRNA expression of hypothalamic neuropeptides at 4 h after i.c.v. rhBMP7, leptin, or vehicle as measured by Q-RT-PCR, showing a significant increase in POMC expression by BMP7 treatment. E) α-MSH immunostaining (brown) of hypothalamic sections 60 min after i.c.v. injection of mice with either vehicle (left panel), rhBMP7 (middle panel), or leptin (right panel), showing increased α-MSH expression in response to both leptin and BMP7 treatments, in the arcuate nucleus. F) Food intake 4 h after i.c.v. BMP7 or vehicle treatment in Agouti mice (KK, Cg-Ay/J), showing that Agouti mice maintain an anorectic response to BMP7 administration. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Figure 5.
BMP7 utilizes the mTOR pathway to regulate food intake. A) Western blot analysis demonstrating that BMP7, when injected i.c.v. in mice, activated multiple signaling pathways in the hypothalamus, most notably the p70S6K and pSTAT3 pathways. Cyclophilin A serves as a loading control. B) Intracerebroventricular rhBMP7-activated phos-p70S6K (brown) in the ARC (left panels) and PVN (right panels), as detected by immunohistochemical staining of hypothalamic sections using a specific antibody against phospho-p70S6K at 30 min following injection of BMP7. Arrows indicate cells that are positive for phos-p70S6K. C) Activation of SMAD and p70S6K signaling pathways by BMP7 in the hypothalamic neuronal cell line N25/2, as evaluated by Western blot analysis using phospho-specific antibodies. D) Western blot analysis of phosphorylated p70S6K in response to 30 min of BMP7 or insulin stimulation in the hypothalamic neuronal cell line N25/2. The 70 kDa-band corresponding to p70S6K is completely blocked by 1 h rapamycin pretreatment. β-Tubulin serves as a loading control. E) Rapamycin, an mTOR inhibitor, reversed the anorectic effect of rhBMP7 injected i.c.v. into mice. Average food consumption at 4 h and 16 h post-treatment are presented. *P < 0.05.
Similar articles
- Role of BMP7 in appetite regulation, adipogenesis, and energy expenditure.
Saini S, Duraisamy AJ, Bayen S, Vats P, Singh SB. Saini S, et al. Endocrine. 2015 Mar;48(2):405-9. doi: 10.1007/s12020-014-0406-8. Epub 2014 Sep 2. Endocrine. 2015. PMID: 25178649 Review. - New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure.
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. Tseng YH, et al. Nature. 2008 Aug 21;454(7207):1000-4. doi: 10.1038/nature07221. Nature. 2008. PMID: 18719589 Free PMC article. - Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation.
Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H. Takahashi M, et al. J Endocrinol. 2008 Dec;199(3):445-55. doi: 10.1677/JOE-08-0226. Epub 2008 Sep 9. J Endocrinol. 2008. PMID: 18780779 - BMP7 improves insulin signal transduction in the liver via inhibition of mitogen-activated protein kinases.
Ma H, Yuan J, Ma J, Ding J, Lin W, Wang X, Zhang M, Sun Y, Wu R, Liu C, Sun C, Gu Y. Ma H, et al. J Endocrinol. 2019 Nov;243(2):97-110. doi: 10.1530/JOE-18-0693. J Endocrinol. 2019. PMID: 31394500 - Hypothalamic mTOR: the rookie energy sensor.
Martínez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Martínez de Morentin PB, et al. Curr Mol Med. 2014 Jan;14(1):3-21. doi: 10.2174/1566524013666131118103706. Curr Mol Med. 2014. PMID: 24236459 Review.
Cited by
- Questioning the preclinical paradigm: natural, extreme biology as an alternative discovery platform.
Buffenstein R, Nelson OL, Corbit KC. Buffenstein R, et al. Aging (Albany NY). 2014 Nov;6(11):913-20. doi: 10.18632/aging.100704. Aging (Albany NY). 2014. PMID: 25553771 Free PMC article. Review. - Comparative transcriptome analysis of hypothalamus-regulated feed intake induced by exogenous visfatin in chicks.
Li Z, Liu X, Zhang P, Han R, Sun G, Jiang R, Wang Y, Liu X, Li W, Kang X, Tian Y. Li Z, et al. BMC Genomics. 2018 Apr 11;19(1):249. doi: 10.1186/s12864-018-4644-7. BMC Genomics. 2018. PMID: 29642854 Free PMC article. - CRISPR-mediated BMP9 ablation promotes liver steatosis via the down-regulation of PPARα expression.
Yang Z, Li P, Shang Q, Wang Y, He J, Ge S, Jia R, Fan X. Yang Z, et al. Sci Adv. 2020 Nov 27;6(48):eabc5022. doi: 10.1126/sciadv.abc5022. Print 2020 Nov. Sci Adv. 2020. PMID: 33246954 Free PMC article. - Brown and beige fat: the metabolic function, induction, and therapeutic potential.
Qian S, Huang H, Tang Q. Qian S, et al. Front Med. 2015 Jun;9(2):162-72. doi: 10.1007/s11684-015-0382-2. Epub 2015 Jan 8. Front Med. 2015. PMID: 25573295 Review. - Targeting adipose tissue in the treatment of obesity-associated diabetes.
Kusminski CM, Bickel PE, Scherer PE. Kusminski CM, et al. Nat Rev Drug Discov. 2016 Sep;15(9):639-660. doi: 10.1038/nrd.2016.75. Epub 2016 Jun 3. Nat Rev Drug Discov. 2016. PMID: 27256476 Review.
References
- Chen D., Zhao M., Mundy G. R. (2004) Bone morphogenetic proteins. Growth Factors 22, 233–241 - PubMed
- Mira H., Andreu Z., Suh H., Lie D. C., Jessberger S., Consiglio A., San Emeterio J., Hortiguela R., Marques-Torrejon M. A., Nakashima K., Colak D., Gotz M., Farinas I., Gage F. H. (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89 - PubMed
- Tobin J. F., Celeste A. J. (2006) Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov. Today 11, 405–411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 DK080058/DK/NIDDK NIH HHS/United States
- F32-DK091996/DK/NIDDK NIH HHS/United States
- UL1 RR 025758-01/RR/NCRR NIH HHS/United States
- P30-DK036836/DK/NIDDK NIH HHS/United States
- T32-DK007260-33/DK/NIDDK NIH HHS/United States
- T32 DK007260/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DK077097/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- F32 DK091996/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous